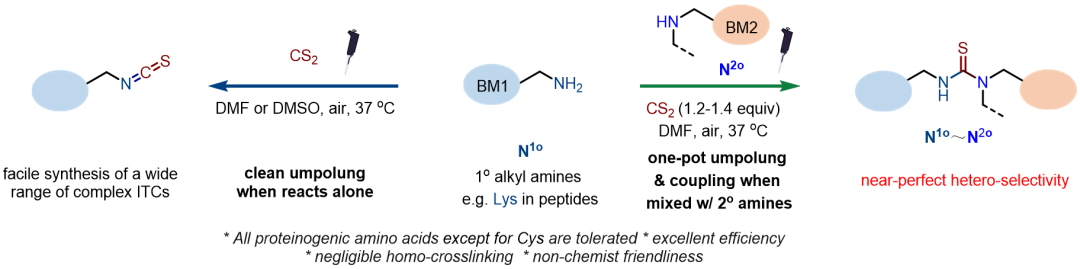
The rapid advancement of modern biotherapeutics demands more powerful strategies for precisely engineering complex biomolecules. In particular, versatile and efficient methods for cross-linking biorelevant molecules (BMs) with both natural and synthetic origins are needed to facilitate the construction of multifunctional bioconjugates, including antibody-drug conjugates, proteolysis-targeting chimeras (PROTACs), and functionalized nanoparticles. Given the scarcity of endogenous electrophilic groups (El) in native biorelevant molecules, bioconjugation mainly relies on cross-linking the more abundant nucleophilic groups (Nu) using suitable linkers. However, achieving selective coupling of polarity-mismatched endogenous handles in complex molecular settings remains a substantial challenge. 1) From a scope perspective, the existing cross-linking strategies are mainly limited to pairing highly reactive but scarce thiol nucleophiles with less reactive nucleophilic groups, mostly amines, to achieve the critical hetero-selectivity required for linking two biorelevant molecules. Thiol-independent methods that can accommodate lower-reactivity nucleophiles are needed. 2) From a practical perspective, current approaches predominantly employ complex extender-type linkers equipped with two distinct tethered electrophilic ends. These methods are not only structurally invasive but also require multi-step operations, often necessitating a significant excess of linkers and intermediate purification steps to ensure efficiency. A promising alternative lies in the development of smart and compact linkers capable of reversing the polarity of the targeted Nu groups via on-site umpolung. Ideally, such linkers should enable the selective umpolung of Nu1 of BM1 and its subsequent coupling with Nu2 of BM2 to proceed in a one-pot reaction.
Recently, Gong Chen’s group have developed a simple yet effective umpolung strategy for biomolecule modification through the isothiocyanation of primary alkyl amine groups using CS2 under ambient aerobic oxidation conditions. These mild conditions are particularly advantageous for complex molecular systems, as they selectively target primary alkyl amines while sparing most other native functional groups, including secondary alkyl amines. The suppression of oxidative side reactions involving secondary amines, combined with their superior nucleophilic reactivity toward isothiocyanates compared to primary amines under mildly basic conditions, enables nearly perfect hetero-selective cross-linking of two biorelevant molecules via their commonly available amino handles in a one-pot operation, offering a streamlined, thiol-independent approach for bioconjugation. Relevant achievements were published in Angew. Chem. Int. Ed., 2025. DOI: 10.1002/anie.202507734.