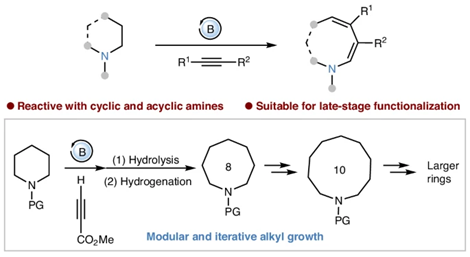
Amines are a vital class of compounds playing crucial roles in pharmaceuticals, agrochemicals, materials and catalysts. Among them, medium-sized cyclic (8–11-membered) and macrocyclic (>12-membered) amine derivatives are particularly promising in pharmaceutical chemistry. Their preorganized conformations, with a balanced flexibility and rigidity, enhance their ability to bind strongly towards biological targets and provide resistance to decomposition under physiological conditions. Despite their promising potential, the utilization of these compounds is hindered by considerable synthetic challenges, limiting their accessibility. For instance, a brief SciFinder survey of simple, unsubstituted cyclic amines reveals that five-, six- and seven-membered cyclic amines are widely available commercially, priced at less than US$0.15 per gram. By contrast, the availability of medium-sized cyclic amines drops sharply, with prices escalating to over US$1,000 per gram for 10- and 11-membered rings. Furthermore, 12-, 14- and 15-membered cyclic amines are available only through custom synthesis from fewer than 4 suppliers, with no pricing information provided. The conventional synthesis of medium-sized cyclic and macrocyclic amines relies on cyclization or cycloaddition of acyclic precursors. These processes are often hampered by kinetic and thermodynamic barriers due to entropy loss and the build-up of destabilizing transannular strain. Furthermore, intermolecular condensation often competes with cyclization, adding further complexity to the process. An emerging strategy to circumvent the challenges is the ring expansion of cyclic amines. This process not only increases entropy but also alleviates transannular strain, offering a promising avenue for synthesis. Nevertheless, this strategy is hindered by the difficulty of cleaving the inert C–N or C–C bonds, a challenging research topic that has received considerable research interest over the past decade. Current studies on ring expansion of cyclic amines have focused primarily on amines having functional groups, where the formation of thermodynamically stable structure via reactions of functional groups provides the driving force for ring opening. By contrast, the ring expansion of unfunctionalized cyclic amines, which could enhance synthetic efficiency by obviating prefunctionalization steps, remains largely underexplored.
Recently, Xiao-Chen Wang’s group have developed a transition-metal-free method for the insertion of alkynes into unfunctionalized amines via functional conversion from C–H to C–C. Demonstrating broad applicability, this method is compatible with various cyclic and acyclic amines, as well as alkynes. It facilitates the synthesis of medium-sized and macrocyclic amines that were previously inaccessible. The utility of this approach in late-stage functionalization offers a pathway to novel drug candidates. Relevant achievements were published in Nat. Chem., 2025. DOI: 10.1038/s41557-025-01849-1.