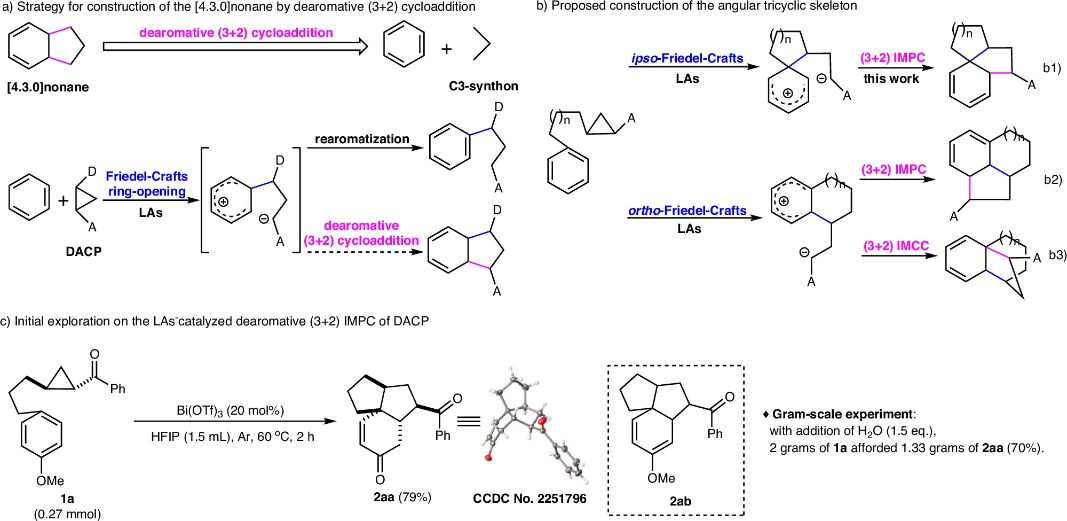
Developing general and highly efficient strategies to construct structurally complex and diverse three-dimensional (3D) polycyclic skeletons from cheap and easily available feedstock is a tempting yet highly challenging topic in organic synthesis and drug discovery. Cyclopentane and cyclohexane are the two most commonly existing cyclic skeletons in natural products and synthetic biologically active molecules. Angular tricyclic and related polycyclic skeletons feature typical cores in an intriguing type of biologically active and structurally complex and novel natural products. These angular skeletons are composed of the cyclopentane and cyclohexane through various topological connection modes. For example, two cyclopentanes form a diquinane [3.3.0]octane skeleton through a fused mode. Via a shared cyclopentane, combination of two [3.3.0]octane skeletons, one [4.3.0]nonane and one [3.3.0]octane skeletons, or two [4.3.0]nonane skeletons afford angular 5-5-5, 5-5-6, and 6-5-6 tricyclic carbocycles respectively. The angular 5-5-5, 5-5-6, and 6-5-6 tricyclic carbocycle and their related polycyclic skeletons exist in several unique biologically active natural products. In these angular tricyclic carbocycles, the three rings share a quaternary carbon center and there are two or more continuous quaternary carbon centers in some natural products. Due to the challenges of efficiently constructing their diverse and congested polycyclic skeletons, as well as their continuous and dense stereochemical centers, such natural products have long attracted organic chemists.
Recently, Zhongwen Wang’s group have developed the LAs-catalyzed dearomative (3 + 2) cycloaddition of DACP with benzene ring. This is also the example of (3 + 2) cycloaddition of a C3-synthon with C = C of benzene. This supplies a general and highly efficient strategy for construction of structurally complex and diverse angular tricyclic and polycyclic carbocycles in natural products. Features of this method also include easy availability of the feedstock and convenient operation. This strategy will demonstrate the potential in the total syntheses of natural products and drug discovery. Relevant achievements were published in Nat. Commun., 2024. DOI: s41467-024-53562-1.