Aromatic compounds with cyclic delocalized (4n + 2) electrons have long been of great interest because of their unique properties. Although the concept has been successfully applied to most organic systems, 2π aromatic systems are mainly confined to charged strained rings such as cyclopropenylium cations and cyclobutadiene dications. For neutral cyclobutane-1,3-diyl four-membered rings (4MRs), the 2e on the 1,3-positions is reluctant to delocalize because of the saturated tetrahedral carbon atoms. In fact, these systems exhibit significant diradical character, and thus are transient species. As such, main group analogues of cyclobutane-1,3-diyl have been investigated extensively for the generation of diradicals. Because of the unique bonding situations and relatively complicated orbital contributions, some main group 4MRs with a significant diradical character have been isolated and structurally characterized. However, none of the 4MRs features a 2e delocalized structure.
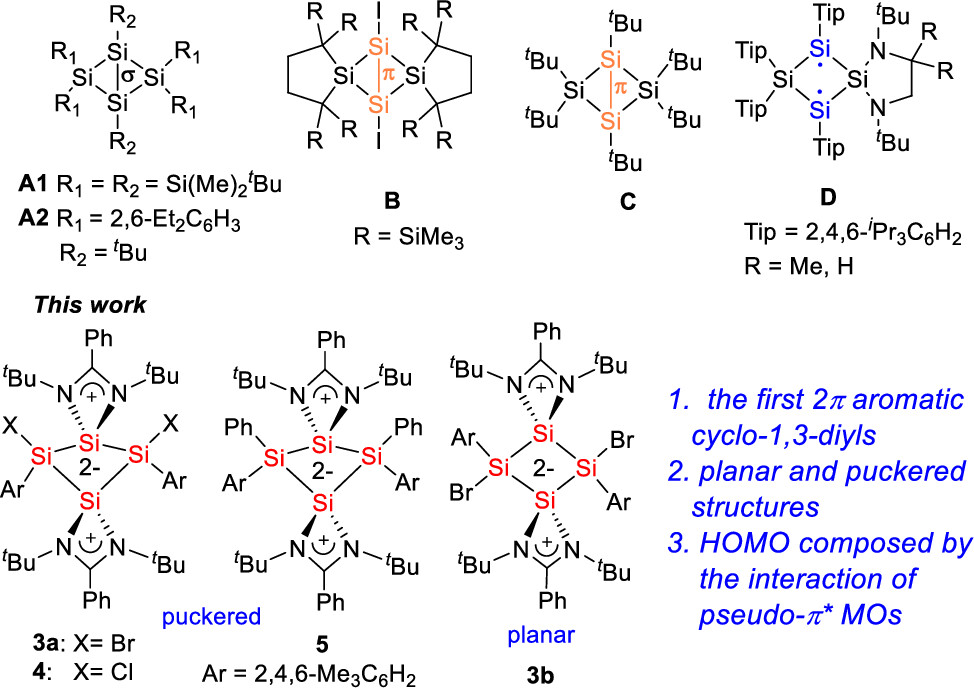
Comparison of Electronic Structures of Si4 Analogues of Cyclobutane-1,3-dilyls
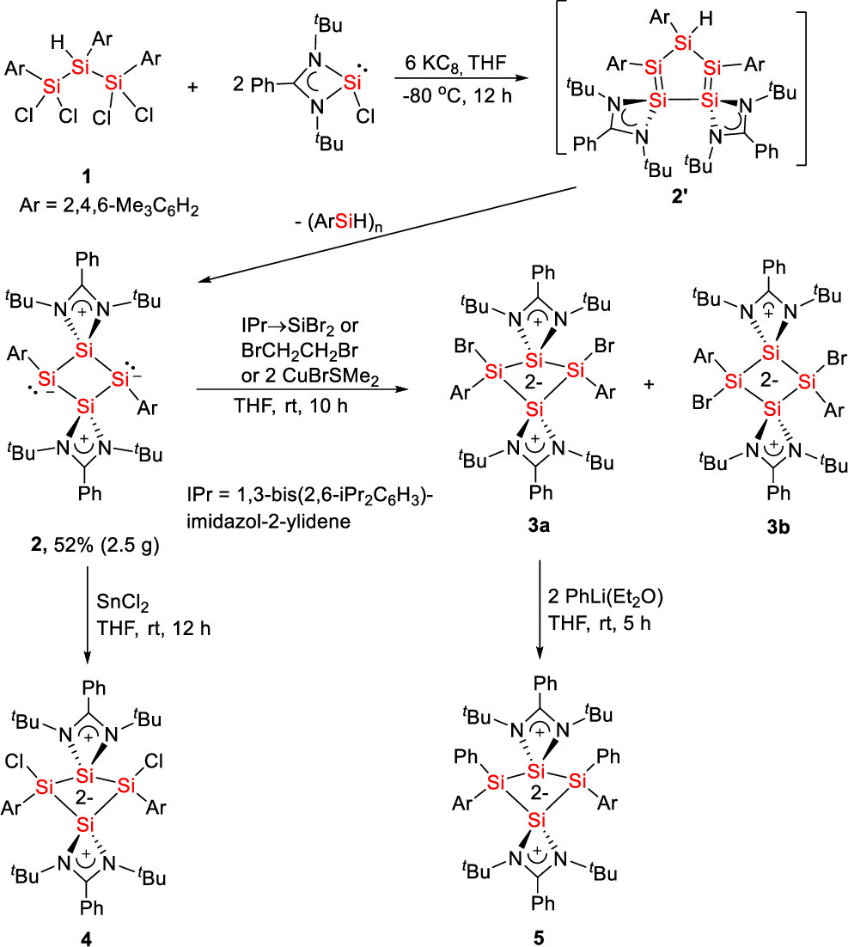
Synthesis of Tetrasilacyclobutadiene 2 and Tetrasilacyclobutane-1,3-diyls 3-5