Alkenes are important raw materials, and the development of methods for their synthesis has long been the focus of chemists. One such method is the Wittig reaction, that is, the reaction of a carbonyl compound with a nucleophile such as a phosphorus ylide, to generate an alkene by means of an addition-elimination process. This method has the advantages of a broad substrate scope, good functional group tolerance, high yields, and operational simplicity, making it one of the most reliable and practical methods for olefin synthesis. However, this protocol is mainly improved toward a practical stereoselective synthesis of thermodynamically stable E-alkenes, while there are still some difficulties in achieving selectivity for the thermodynamically unstable Z configuration, especially for the synthesis of trisubstituted Z-alkenes with ketones as substrates The control of Z-selectivity of this strategy is mainly achieved by changing the substituents of carbonyl substrates, activity of phosphorus ylides, reaction conditions, and ionic additives. Nevertheless, these methods are only well applicable to aldehyde substrates to obtain Z-disubstituted olefins, while the Z-selective Wittig reaction of ketones can be achieved only with active alkyl phospholipids. Therefore, it is necessary to develop reliable strategies for the synthesis of Z-trisubstituted alkenes, which is of great importance in the synthesis, functionalization, and modification of natural products and drug molecules.
Recently, Shou-Fei Zhu’s group we reported a method for Wittig/B─H insertion reactions by which a series of thermodynamically unstable trisubstituted Z-boryl alkenes could easily be synthesized from readily available dialkyl ketones, diazomethyl phosphate or trimethylsilyldiazomethane, and Lewis base–borane adducts under mild reaction conditions with good functional group tolerance. Various previously unavailable trisubstituted Z-alkenes were prepared through the stereospecific transformations of boryl groups of the products. In addition, intramolecular Wittig/B─H insertion reactions smoothly produced five- to eight-membered B─N or B─P heterocyclic compounds, many of which cannot be synthesized by known methods. Relevant achievements were published in Sci. Adv. 2023, DOI: 10.1126/sciadv.adj2486.
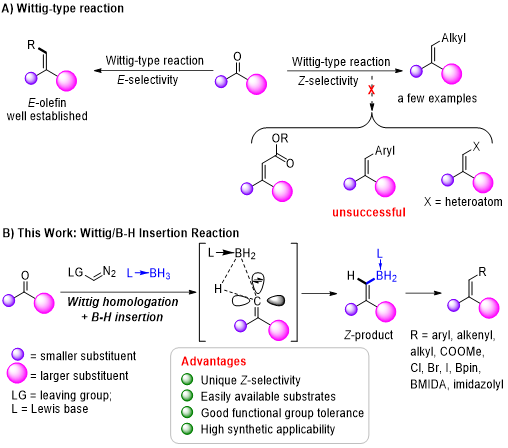
Fig. Accesses to boryl alkenes.
(A) Wittig-type reaction. (B) This work: Wittig/B─H insertion reaction.