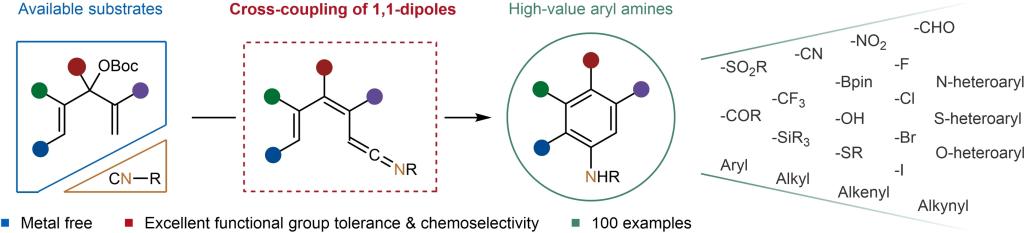
Aryl amines are ubiquitous in natural products, pharmaceuticals and organic materials, and their synthesis by nitro reduction, Curtis, Schmidt, and other rearrangement strategies is a well-established and ever-improving field. The Ullmann-Ma, Buchwald–Hartwig, Chan–Lam, and other cross-coupling reactions have revolutionized this field and are extensively employed in industry. In addition, electro-/photochemical aromatic C−H amination offers an economical approach to constructing these essential scaffolds. Despite their versatility, these reactions necessitate specific substrate pre-functionalization, which can be challenging due to the intrinsic reactivity profile of aromatics, such as uncontrollable site selectivity, over activation, and the generation of inseparable mixtures of regio-isomers. Furthermore, the site selectivity of fused (hetero) aromatics is an almost intractable challenge in the absence of a specific directing group. Conversely, de novo synthesis of aryl amines can circumvent the selectivity issues of aromatic chemistry through the design of rational substrates. The Dötz reaction is prized for accessing densely functionalized aromatic compounds with excellent chemo- and regioselectivity. However, the aminobenzannulation is frequently accompanied by the formation of indenes and cyclobutenones and exhibits limited functional group tolerance. Although the multi-step synthesis of chromium complexes can solve the problem to some extent, the inevitable use of stoichiometric unstable organolithium reagents and toxic chromium complexes have restricted its further development, so there is still an urgent need for more concise and safer synthetic strategies.
Recently, You Huang’s group presented a novel metal free Dötz-type aminobenzannulation reaction that circumvents the selectivity issues inherent in aromatic chemistry, as well as the use of stoichiometric unstable organolithium reagents and toxic chromium complexes. The concept of utilizing readily available isocyanides and Morita–Baylis–Hillman (MBH) carbonates to achieve 1,1-dipoles cross-coupling to construct ketenimine is the key to success, which has been experimentally and computationally verified. The tandem 6π-electrocyclization/aromatization process offers a versatile method for synthesizing functionalized anilines, fused aryl amines and fused heteroaryl amines. Relevant achievements were published in Angew. Chem. Int. Ed., 2023, DOI: 10.1002/anie.202310133