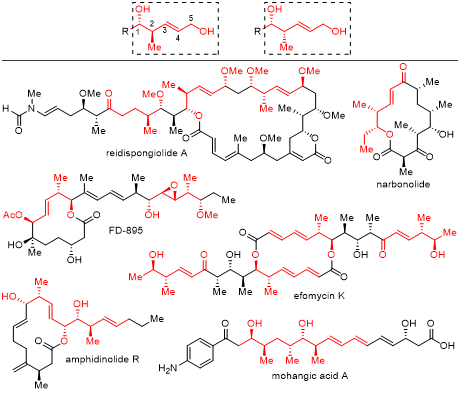
Enantioenriched homoallylic alcohol 2-methyl-3-pentene-1,5-diols are essential components found in many natural products, and their reduced or oxidized forms also exhibit biological significance. These compounds contain an allylic alcohol motif that can be transformed into various functional groups, rendering them versatile precursors in C–C bond-forming reactions. However, current synthetic approaches for 2-methyl-3-pentene-1,5-diols generally involve complex multistep processes utilizing chiral starting materials to construct multiple stereogenic elements separately. Despite significant advancements, multiple manipulations are still required, prompting the need for a direct method to access chiral 2-methyl-3-pentene-1,5-diols in one step, which remains a highly desirable objective.
Recently, Li-Jun Xiao & Qi-Lin Zhou’s group presented the first enantioselective nickel-catalyzed borylative coupling of 1,3-dienes with aldehydes, providing an efficient route to highly valuable homoallylic alcohols in a single step. The reaction involves the 1,4-carboboration of dienes, leading to the formation of C–C and C–B bonds accompanied by the construction of two continuous stereogenic centers. Enabled by a chiral spiro phosphine-oxazoline nickel complex, this transformation yields products with exceptional diastereoselectivity, E-selectivity, and enantioselectivity. The diastereoselectivity of the reaction can be controlled by employing either (Z)-1,3-dienes or (E)-1,3-dienes. Relevant achievements were published in J. Am. Chem. Soc., 2023, DOI: 10.1021/jacs.3c07697
Learn More:
Diastereodivergent and Enantioselective Synthesis of Homoallylic Alcohols via Nickel-Catalyzed Borylative Coupling of 1,3-Dienes with Aldehydes
Jin-Tao Ma, Tianze Zhang, Bo-Ying Yao, Li-Jun Xiao*, Qi-Lin Zhou
https://pubs.acs.org/doi/10.1021/jacs.3c07697