Nonbenzenoid polycyclic aromatic hydrocarbons (PAHs), which contain nonhexagonal rings, have attracted broad interest owing to their appealing topological structures, unique properties, and applications in optoelectronic devices. In recent years, a number of novel nonbenzenoid PAHs have been synthesized either in solutions or on metal surfaces. The remarkable synthetic achievements have enabled extensive investigations of the properties of these novel compounds, such as (anti)aromaticity,4 open-shell characters, and chiroptical responses. Furthermore, as exemplified by azulene derivatives, the optoelectronic applications of nonbenzenoid PAHs have also been demonstrated. However, the semiconducting properties of other nonbenzenoid PAHs beyond azulene have been rarely exploited. Besides, the advantages of nonbenzenoid PAHs over their benzenoid counterparts in optoelectronic materials have not been clearly revealed.
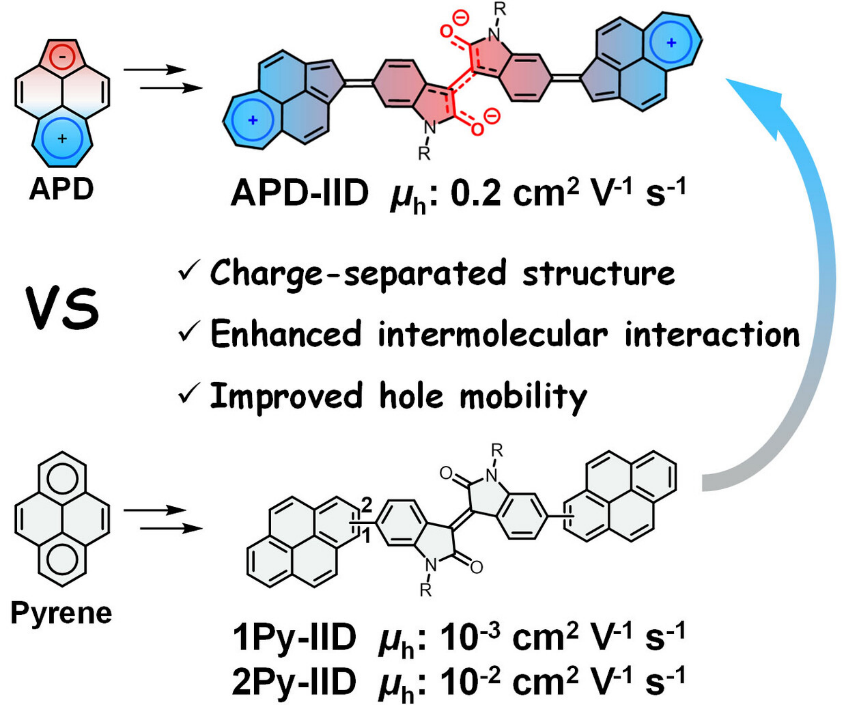
Acepleiadylene (APD), which is a classical nonbenzenoid PAH, features a larger molecular dipole, a smaller optical gap, and a charge-separated structure as compared with its benzenoid isomer pyrene. These characters can introduce novel properties to APD-based materials. For example, the large molecular dipole and the charge-separated structure would be conducive to strong intermolecular interactions and electronic coupling, which can lead to efficient charge transport. As the benzenoid isomer of APD, pyrene has been widely applied as a building block in optoelectronic materials, whereas APD has never been exploited in this regard. Therefore, it is still unclear yet whether APD exhibits any superiority over pyrene in optoelectronic materials.
Recently, Xiao-Ye Wang’s group employed APD as a building block in organic semiconducting materials for the first time, and unravel the superiority of nonbenzenoid APD in electronic applications. They have synthesized an APD derivative (APD-IID) with APD as the terminal donor moieties and isoindigo (IID) as the acceptor core. Theoretical and experimental investigations reveal that APD-IID has an obvious charge-separated structure and enhanced intermolecular interactions as compared with its pyrene-based isomers. As a result, APD-IID displays significantly higher hole mobilities than those of the pyrene-based counterparts. These results imply the advantages of employing APD in semiconducting materials and great potential of nonbenzenoid polycyclic arenes for optoelectronic applications. Relevant achievements were published in Angew. Chem. Int. Ed., 2023, DOI: 10.1002/anie.202306509.