In view of the deeper understanding of molecule design, device engineering and charge transfer/transport mechanism, organic solar cells (OSCs) have undergone a blowout growth in the past few decades, leading to power conversion efficiencies (PCEs) of single-junction OSCs surpassing 19% and tandem OSCs over 20%, respectively. At present, the continuous exploration of small molecule acceptors (SMAs) with a distinctive “acceptor-donor-acceptor” (A-D-A) architecture is still the most concerned issue if more efficient OSCs are further expected. Note that the desirable three-dimensional (3D) intermolecular packing network, which will be in favor of superior charge generation/transport/recombination dynamics, is highly desired for well-established SMAs. Among the various strategies to tune molecule packing, halogenation on end units of SMAs has been the most employed but also indispensable one, especially in the state-of-the-art OSCs. As it has been proven that halogenation plays a crucial role in (1) greatly tuning molecular energy levels; (2) enhancing intermolecular charge transfer and intermolecular packings simultaneously; (3) improving molecular crystalline ordering and film morphologies; (4) contributing to superior charge carrier transport behaviors, suppressed charge recombination and upgraded photovoltaic parameters, etc. In addition, theoretical studies have also revealed that halogenation on SMAs is inclined to enlarge the variation of dipole moments between ground and excited states of SMAs, thus minimizing molecular exciton binding energies and endowing with SMAs highly efficient exciton dissociation even driven by a quite small energy offset.
Recently, Yongsheng Chen’s group constructed three A-D-A type SMAs (CH20, CH21 and CH22) with delicately brominating on the central unit rather than conventional end groups. In this way, the unique advantages of bromides, like high crystallinity, easily polarizing, efficient halogen bonding interaction, etc., are maximized while circumventing the adverse effect of large steric hindrance on molecular packing, especially for the “E/E” packing mode. A systemic investigation has revealed that brominating on the central unit of CH-series SMAs could endow with CH22 increased polarizability and enlarged dipole moment, and thus improved relative dielectric constant. In addition, the large atomic radius of bromine on the central unit of SMAs transforms the intermolecular packing mode from “C/C” to the more effective “E/E” mode, leading to a more compact and ordered intermolecular packing and also superior 3D molecular packing network for CH22. More excitingly, the temperature-dependent PL measurement unveils that CH-series SMAs not only possess greatly reduced Eb with respect to the state-of-the-art Y6 but also make it high potential for achieving a much smaller Eb even comparable to inorganic semiconductors if further halogenation on the central unit is performed to delicately tuning both molecular structures and interocular packing modes. As a result, due to the facilitated charge separation/transport dynamics, PM6:CH22-based binary OSCs eventually render the highest efficiency of 19.06% for brominated SMA-based devices, along with good stability. Moreover, a record-breaking efficiency of 15.70% was also achieved when further thickening active layers up to 500 nm, which is highly important to the large-scale production of OSCs. Relevant achievements were published in Nat. Commun., 2023, DOI: 10.1038/s41467-023-40423-6.
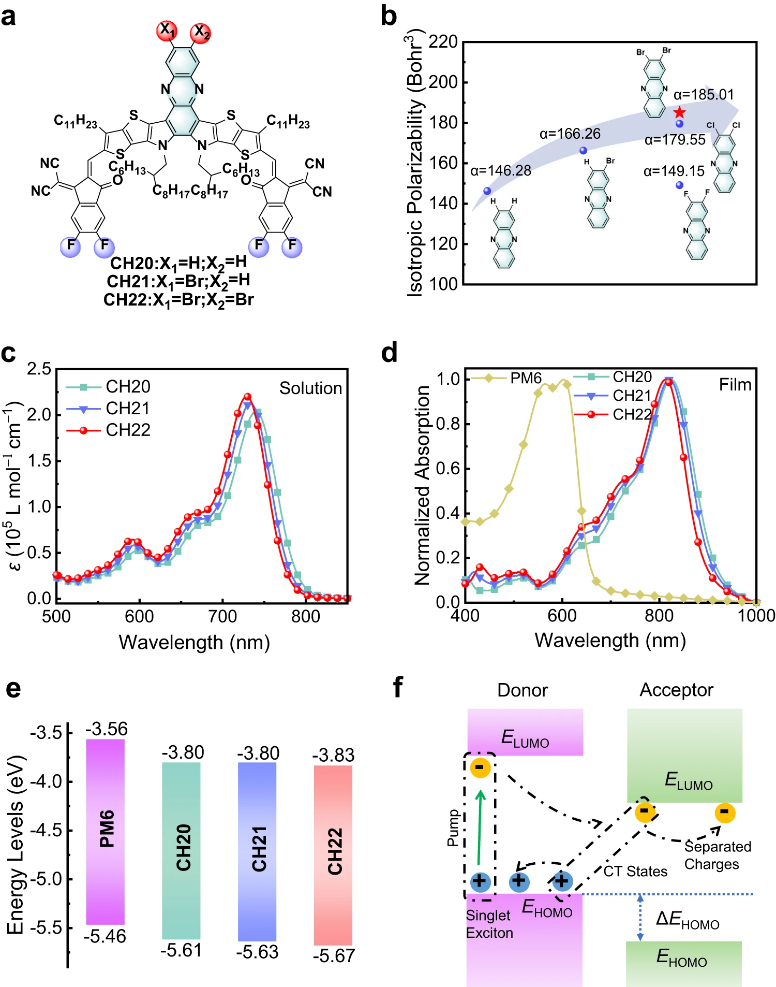
a Chemical structures of CH20, CH21 and CH22. b The isotropic polarizability. c Electron absorption spectra of CH20, CH21 and CH22 dissolved in dilute chloroform. d Normalized absorption spectra of neat films for PM6, CH20, CH21 and CH22. e Energy level diagram of PM6, CH20, CH21 and CH22 neat films derived from CVs. f Working mechanism diagram of free carrier generation at donor-acceptor interfaces.