The first sulfonamide antibacterial drug, Prontosil, was discovered in 1932, and sulfonamide groups occur frequently in clinically relevant synthetic drugs, including antibiotics, diuretics, anticancer agents, and antipsychotic drugs. Aryl sulfonamides are particularly valuable because they show a unique physicochemical profile, are chemically and metabolically stable, and are rich in heteroatoms. These advantages have combined to propel the use of aryl sulfonamide groups to improve drug efficacy and bioavailability. The traditional approach to the synthesis of these valuable structural motifs involves combining amines with sulfonyl chlorides; however, the success of this approach depends on the stability and availability of sulfonyl chlorides, the nucleophilicity of amines, and the functional group compatibility of the reaction conditions. Functionalized sulfonyl chlorides can be prepared by electrophilic chlorosulfonylation of arenes or by oxidative chlorination of aryl organosulfur compounds, but these methods have limited functional group compatibility and suffer from limitations imposed by the need for site-selective functionalization, strongly acidic conditions, and odorous thiol substrates. Moreover, many of the reported nucleophilic substitution reactions between electron-deficient amines and functionalized sulfonyl chlorides are extremely inefficient for the preparation of bioactive sulfonamides and require harsh conditions. These issues could be avoided if an efficient route for the synthesis of sulfonamides was developed from abundant, inexpensive, and readily available reagents.
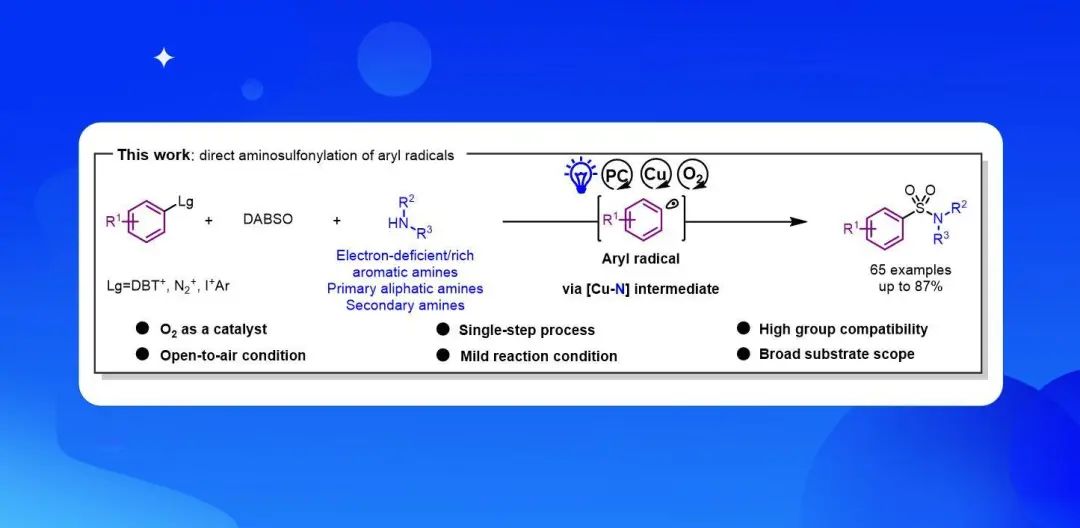
Recently, Qingmin Wang’s group developed a method for direct aminosulfonylation reactions involving readily available aryl radical precursors (aryl dibenzothiophenium, aryldiazonium, and diaryliodonium salts) for the single-step synthesis of sulfonamides by synergetic photoredox and copper catalysis. This novel method leverages the capture of sulfonyl radical intermediates by a Cu(II) amido complex, has good functional group compatibility, and is applicable to a broad range of substrates, including electron-deficient amines. The method also was used for efficient syntheses of sulfanitran and an N-aryl sulpiride derivative. Mechanistic studies support an oxygen-catalyzed process in which O2 in air oxidizes a Cu(I) complex to generate a copper peroxo complex. fac-IrIV(ppy)3+l oxidizes the complex to close the photocatalytic cycle. Our findings give insight into metallaphotoredox catalysis and the role of O2 (air) in the mechanism. Against the backdrop of increasing interest in research on aryl radicals, this practical method rapidly and efficiently transforms aryl radicals into sulfonamides. The method can be expected to facilitate chemical biology research and drug discovery. Relevant achievements were published in ACS Catal., 2023, DOI: 10.1021/acscatal.3c03096.