Fluorine-containing compounds have unusual physicochemical properties and have had a considerable impact on the discovery of new medicines, agrochemicals, catalysts, and functional materials. Thus, the development of fluorine-containing building blocks has recently been receiving increasing attention. The gem-difluoromethylene group is considered to be a bioisostere of carbonyl groups and oxygen atoms of ethers and can modulate the pKa of neighboring functional groups.gem-Difluoroalkyl groups (–CF2–R) are key moieties in many fluorine-containing drugs, including lubiprostone, oteseconazole, vinflunine, and gemcitabine. The introduction of a gem-difluoroalkyl group into bioactive molecules is an effective strategy for studying structure–activity relationships and tuning the pharmacological activity of drugs and drug candidates. The efficient construction of chiral gem-difluoroalkyl compounds has attracted substantial research interest over the past few decades. However, the types of chiral gem-difluoroalkyl compounds are still limited in number because of lack of efficient synthetic methods. Since organoboron compounds, alkynes, and allenes are common building blocks in organic synthesis, gem-difluoroalkyl-substituted chiral boron compounds and allenes are expected to become novel chiral gem-difluoroalkyl reagents. To our knowledge, there is only one catalytic method for the synthesis of boron-substituted chiral gem-difluoroalkyl compounds, while chiral gem-difluoroalkyl propargylic borons and chiral gem-difluoroalkyl-substituted allenes remain unknown. Therefore, the development of efficient, convenient methods for the synthesis of easily transformable chiral gem-difluoroalkyl-substituted boron compounds bearing alkyne and allene motifs would be highly desirable.
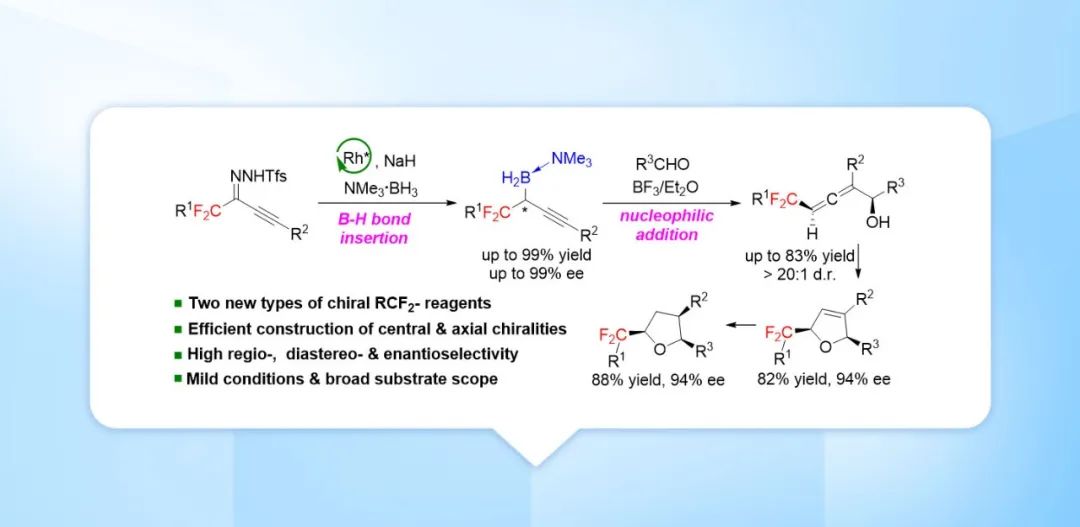
Recently, Shou-Fei Zhu’s group report a method for dirhodium-catalyzed B–H bond insertion reactions using gem-difluoroalkyl alkynyl N-triftosylhydrazones as carbene precursors for the preparation of a wide range of novel, stable chiral gem-difluoroalkyl propargylic borons in high yields with high enantioselectivities. They also developed a method for BF3·Et2O-promoted allenylation of aldehydes with a chiral gem-difluoroalkyl propargylic boron; this method offers rapid access to a wide range of chiral gem-difluoroalkyl α-allenols with adjacent axial and central chiralities. These two types of chiral gem-difluoroalkyl reagents, which contain easily transformable boron and alkynyl or allenyl moieties, have high value for facilitating the rapid, modular construction of chiral molecules containing gem-difluoroalkyl groups. We demonstrated the synthetic potential of the gem-difluoroalkyl α-allenols by transforming one of them into chiral gem-difluoroalkyl 2,5-dihydrofuran and tetrahydrofuran derivatives. Relevant achievements were published in Chem. Sci., 2023, DOI: 10.1039/D3SC03266C