Based on the metal element with the highest content in the crust, organic aluminum has high stability and can undergo various transformations. It is an important class of organometallic reagents and is widely used in synthesis. The hydroaluminization of alkynes can synthesize more useful alkenyl aluminum from easily available aluminogens, which has important application value.
Recently, Shou-fei Zhu 's group reported firstly the case of iron catalyzed hydroaluminization of alkynes. Using the complex catalyst of 2,9-diaryl-1,10-phenanthroline and iron with well-defined structure, the common commercial DIBAL-H was used as the aluminum reagent to achieve the high efficiency of aryl alkyl substituted for internal acetylene, alkenyl alkyl substituted internal acetylene, amine oriented propargyl amine and diaryl internal acetylene with high stereoselectivity and high regioselectivity of hydroaluminization reactions. Compared with other metal catalysts reported in the literature, the iron catalyst developed in this paper has the following advantages: wide range of substrates, good functional group tolerance, high selectivity, mild conditions, etc., which has good application potential. This reaction takes advantage of the easy conversion of C-Al bond, providing an efficient synthesis method for highly selective synthesis of a variety of trisubstituted olefins. Relevant achievements were recently published in Chemical Science, DOI: 10.1039/d2sc02160a.
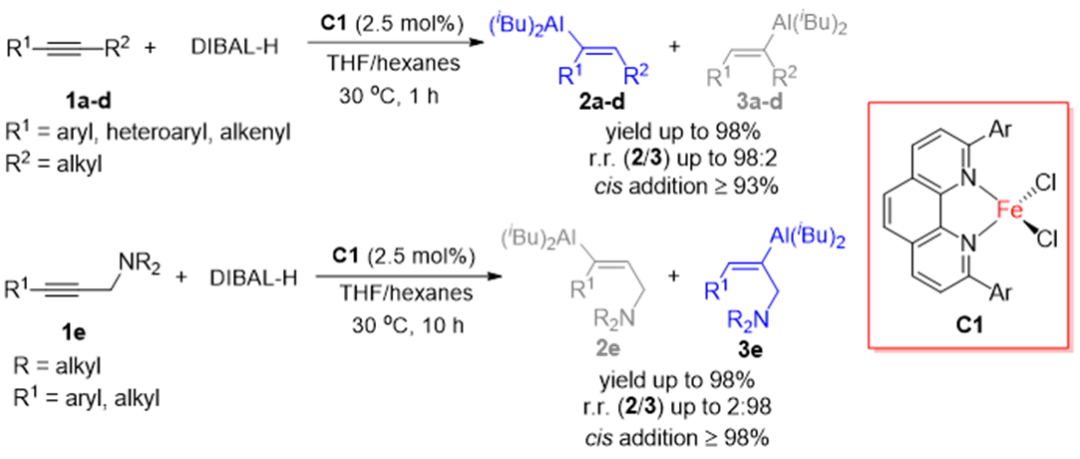
Figure 1. Iron catalyzed hydroaluminization of internal acetylene
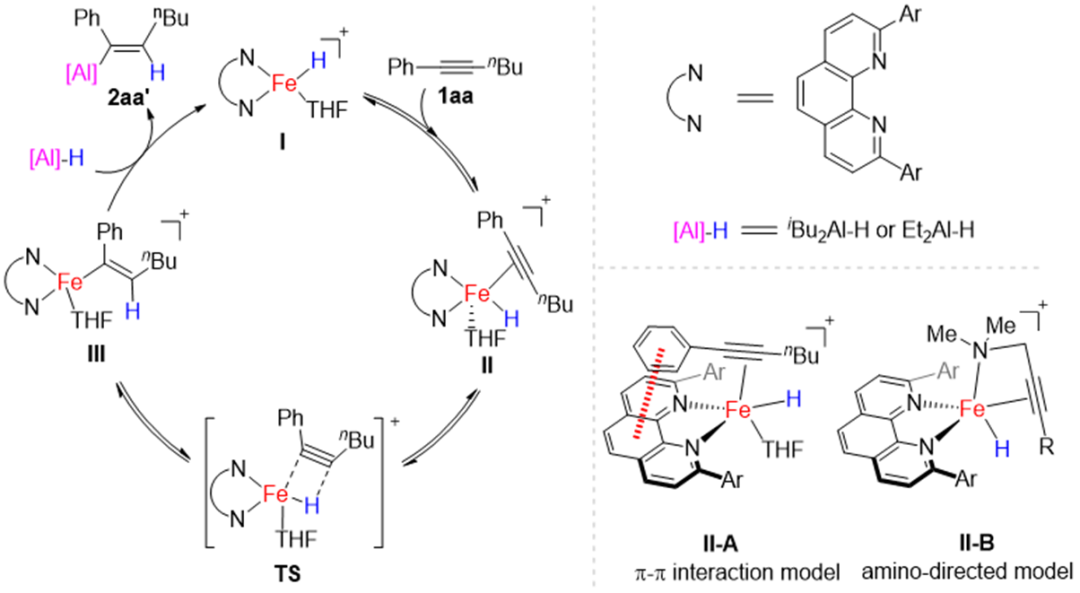
Figure 2. Proposed mechanism