Olefin moieties are present in a wide variety of pharmaceuticals, agrochemicals, and polymers and other materials, and alkene functionalization is one of the most useful reactions for efficiently increasing molecular diversity and complexity. In addition, reactions that involve the migration of aryl groups, such as the Smiles rearrangement, are powerful tools for the synthesis of (hetero)aromatic structural motifs that are unavailable by other methods.
It is a good method to bifunctionalize olefins by aryl migration, which can rapidly synthesize high value-added compounds with multiple functional groups while it is difficult to construct by conventional methods. Recently, professor Qingmin Wang’s group reported a photocatalytic reaction of aromatic group migration from nitrogen or oxygen to carbon for the sulfonyl arylation of inactive olefins. The reaction converts aromatic ethers or aromatic amines with inactive olefins into structures with arylpropanols or arylpropamines, and the reaction has the advantages of using cheap organic photocatalysts, high atom economy, good functional group tolerance, etc. Relevant achievements were published in Green Chem. DOI: 10.1039/d2gc03187f
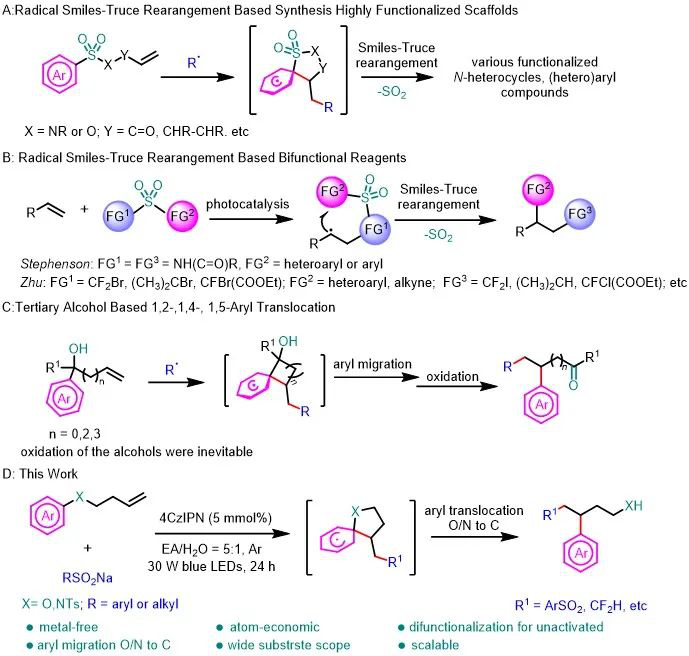
Figure 1. Research background and work summary