Recently, Prof. Chen Gong's group has developed an upgraded version of am-a-gu reaction by broadening and optimizing the cross-linking chemistry of formaldehyde polycomb to achieve the "suture" modification of unprotected peptides, and constructed more complex, novel and topological polycyclic peptide molecules, which has enriched the chemical space of complex peptides. The work was recently published in the Journal of the American Chemical Society (DOI: 10.1021/jacs.2c04620).
Structural constraints on linear peptides by means of chemical modifications are a hot topic in the field of peptide medicinal chemistry in recent years. Among them, macrocyclization is one of the most effective means of peptide modification, and the formation of polycyclic cyclic peptides by cross-linking multiple side chains on linear peptides also provides new ideas for the construction of bioactive molecules with novel topologies. The current strategy for the preparation of polycyclic peptides usually involves the attachment of prefabricated template molecules to the side chains of linear peptides, and this "grafted" polycyclization strategy is mostly "monopolized" by the highly reactive cysteines. Therefore, there is a need to develop new complex polycyclic peptide synthesis strategies with high flexibility and wide applicability (Figure 1a).
Figure 1. Chemoselective modification of constructed polycyclic cyclic peptides
In 2021, Chen's group developed a simple and practical strategy for "binding" natural peptides by using a simple formaldehyde molecule (a) to cross-link lysine (Lys, K) and tyrosine (Tys, Y) and lysine (Lys, K) and arginine (Arg, R) ( Angew. Chem. Int. Ed. 2021, 60, 6646-6652), named the KaY and KaR reactions, respectively. In the KaR reaction, the researchers found that formaldehyde could be selectively and efficiently condensed with guanidine and amino groups to form tetrahydrotriazine (THT) (Figure 1b). On this basis, the group further explored this multi-component condensation reaction.
Recently, Prof. Gong Chen's lab has upgraded and expanded the KaR reaction to a novel polycyclization method with broad substrate versatility and high flexibility using a three-component reaction of amine-formaldehyde-guanidine (am-a-gu, am for amine and gu for guanidine). By using guanidine-based reagents and simple aqueous formaldehyde solutions, the authors were able to "suture" the unprotected peptide under mild conditions to obtain a novel structure of thickened tetrahydrotriazine ring (Fused-THT) and to construct complex and novel polycyclic peptide structures (Figure 2).
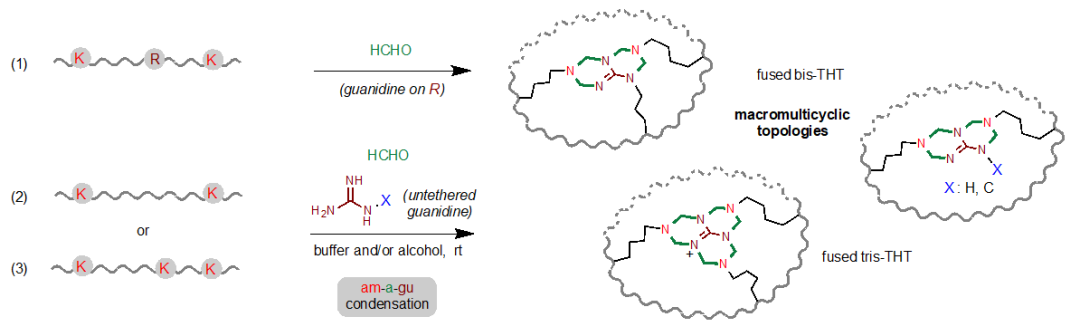
Figure 2 Amine-formaldehyde-guanidine three-component reaction for the construction of polycyclic peptides
In substrate 1, the authors introduced two Lys and one Arg in the sequence, and with HFIP as the solvent and the addition of formaldehyde, a thickened tetrahydrotriazine ring-linked structure was efficiently constructed in 5 h at room temperature, and a pair of bicyclic polypeptides with regional heterostructures was obtained (trace-2), and such multicomponent reactions were named K(2)-a-R reactions, and the reactions also proceeded smoothly in alkaline PBS buffer (trace-4).
In generality studies, most of the polar functional groups do not interfere with the reaction, such as carboxyl (Glu), hydroxyl (Thr), imidazole (His), and sulfhydryl (Cys) can be adjusted by the sequence and thus circumvent their interference with the reaction (9). The yield was low when the N-terminal glycinamide was used as the ring-forming component for GK-a-R ring formation (12), mainly as a monocyclic product (13); while the yield was significantly improved when the N-terminal was replaced with a longer 6-aminohexanoic acid (14). The authors could also successfully apply the K(2)-a-R reaction to the construction of tricyclic polypeptides with a nested topology (11).
figure 3 K(2)-a-R reaction
Then the authors removed the guanidine part (Arg) fixed on the peptide chain in the K(2)-a-R reaction and introduced guanidine hydrochloride as the guanidine component, which also realized the multicomponent condensation reaction and constructed a series of cyclic peptide molecules. formaldehyde and guanidine cross-linked the two Lys on the peptide to construct the cyclic peptide reaction, which the authors named as K(2)-a-gu reaction (gu stands for guanidine).
As shown in Figure 4, a single region isomeric cyclic peptide product can be efficiently obtained by adding guanidine hydrochloride and formaldehyde to a linear peptide chain containing two Lys as a template and using TFE as a solvent for 5 h at room temperature.16 Also the reaction can be carried out in a mixture of water and TFE, and some of the substrates with good water solubility can be carried out smoothly in PBS buffer solution. The reaction can be compatible with almost all amino acid species and the length of the two Lys spacing does not affect the reaction very much. Various guanidine derivatives such as alkynylguanidine, benzylguanidine (32), dansylguanidine (33), and cyclic guanidine (35) can also be involved in the K(2)-a-gu reaction with high efficiency. In addition the reaction can also be carried out efficiently on the solid phase, which avoids intermolecular cross-linking side reactions due to the high concentration in the liquid phase reaction and at the same time lays the groundwork for the high throughput synthesis of these cyclic peptide molecules.
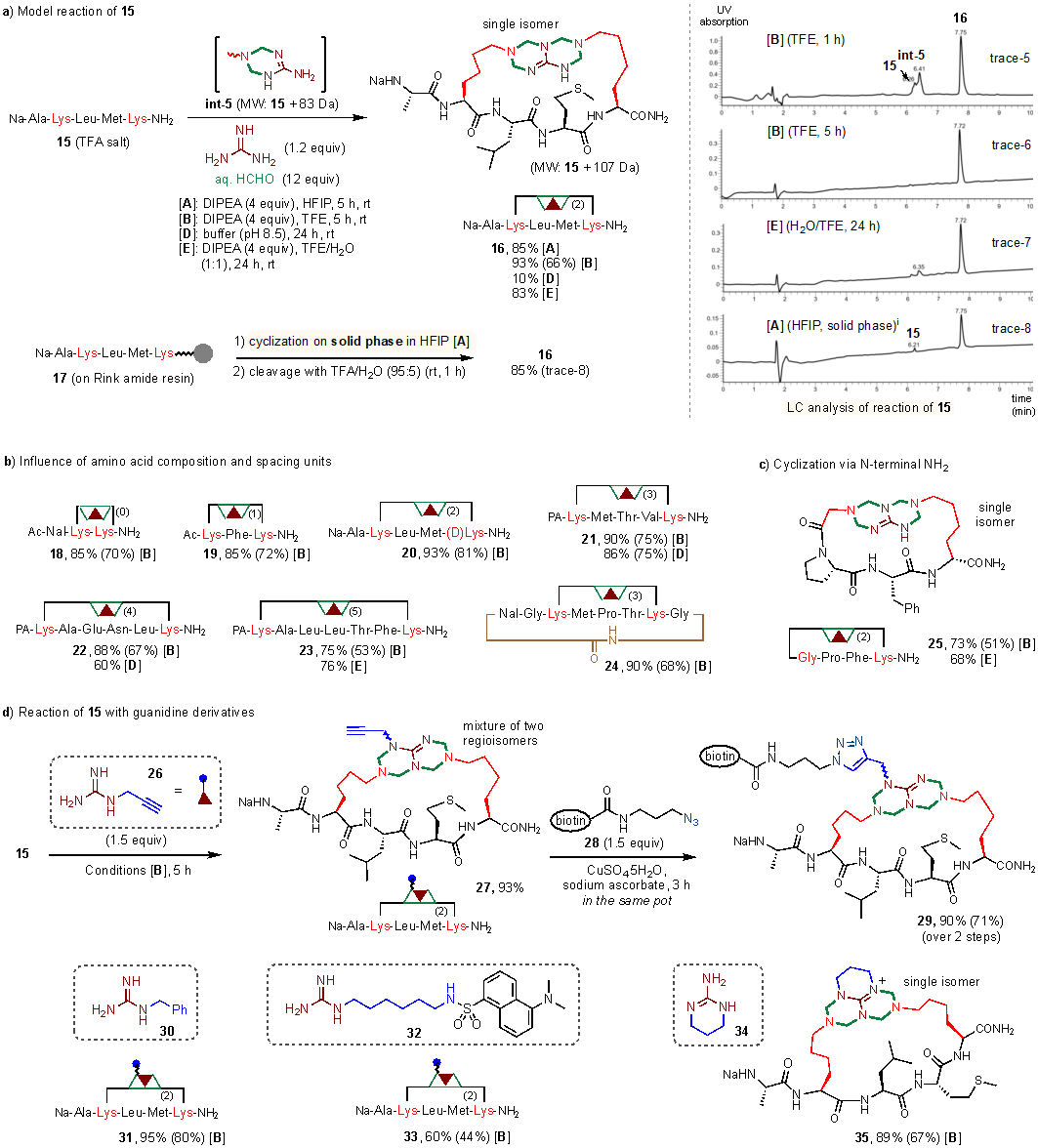
figure 4 K(2)-a-gu reaction
Inspired by the cyclic guanidine product35, the authors then used formaldehyde and guanidine hydrochloride to close the three Lys on the linear peptide chain to efficiently obtain the tris-THT structure and "stitch" the product.37 The authors cross-linked formaldehyde and guanidine with the three Lys on the peptide to construct the bicyclic peptide. The reaction was named as K(3)-a-gu reaction.
This reaction is likewise compatible with various polar amino acids (Glu, Asn, Thr, etc.). In addition, the cyclic peptide, also using lactam-formed cyclic peptides, then underwent the K(3)-a-gu reaction thereby obtaining a product with a bird's nest-like topology.48 Finally, the authors used a one-pot method to obtain a cyclic peptide with a bis-THT structure by first employing the K(2)-a-gu reaction followed by a secondary condensation with another molecule of butylamine to obtain a cyclic peptide with a hetero-tris-THT structure.49
figure 5 K(3)-a-gu reaction
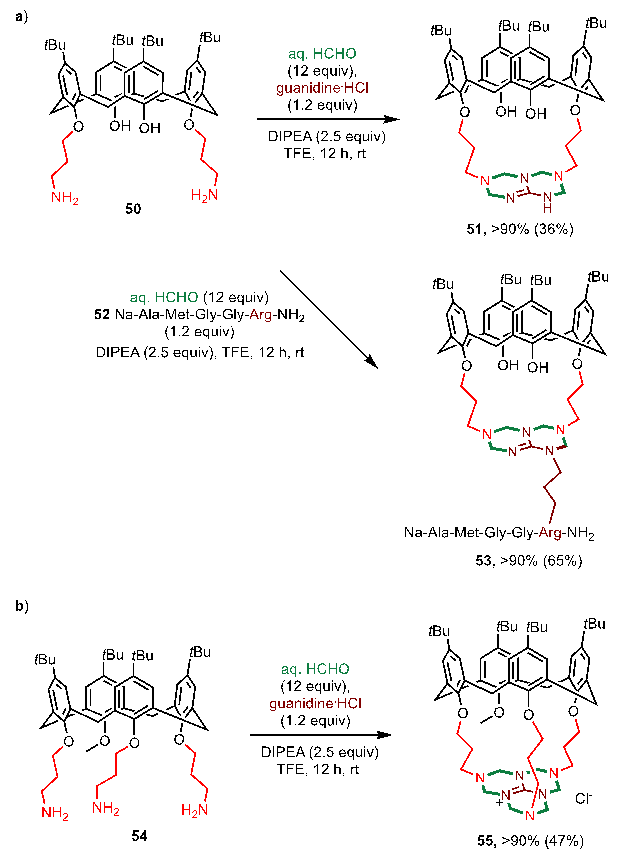
To demonstrate the potential of such reactions, the group of Chen Gong, in collaboration with the group of Guo Dongsheng at Nankai University, used the hydrophilic "caps" of bis-THT and Tris-THT produced by these three-component reactions to graft onto the lower rim of the cup aromatics (51 and 55), thus forming cavities of a certain size. 1H NMR titration experiments on the complexation of halo-negative ions (F-, Cl-, Br-) revealed that the product (55) obtained from this thickened tetrahydrotriazine ring modification was selectively bound to F- (3.64 × 103 M-1) in deuterium chloroform at room temperature, while the unformed Tris-THT structure 54 had a weaker binding capacity to F- (Ka=1.0). (Ka=1.60 × 102 M-1).
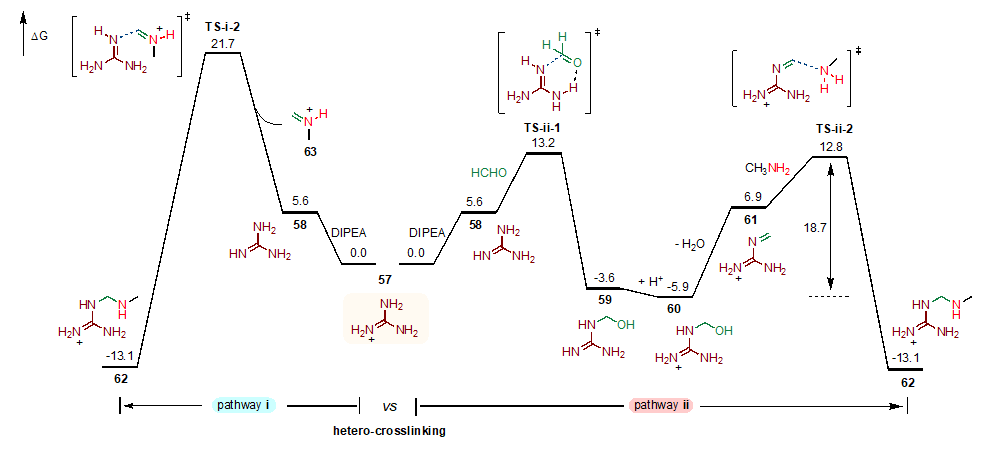
In the part of theoretical calculation, Xue Xiaosong's group at the Institute of Organic Sciences, Chinese Academy of Sciences, revealed the reaction process by density flooding theory (DFT) (Figure 7). Firstly, the reaction system was simulated with the reaction between formaldehyde, methylamine and guanidine, and the two possible initiation paths for the initial reaction were theoretically calculated, and the results showed that the initiation step in pathway II was kinetically more favorable. The protonated guanidinium group57 undergoes deprotonation and continues to react with formaldehyde to produce the hydroxymethylated product of guanidine.59 It is then protonated and dehydrated to obtain the guanidinium imide product.61 At this point, methylamine acts as a nucleophilic reagent to attack the guanidinium imide and crosses the 18.7 kcal/mol energy barrier to produce the intermolecular cross-linking product in the first step, which is able to release enough energy (ΔG= -13.1 kcal/mol) to provide a strong driving force for the completion of the whole reaction provided a strong driving force. Meanwhile, the authors found that the homo-crosslinking products of guanidine and amine were higher in energy than the heterogeneous crosslinking products, which provided a theoretical basis for the preferential and selective occurrence of heterogeneous crosslinking in polyamino systems.
In summary, by broadening and optimizing the cross-linking chemistry of formaldehyde, Prof. Chen Gong's group has developed an upgraded version of the am-a-gu reaction based on previous work to achieve "suture" modification of unprotected peptides, and constructed more complex, novel and topological polycyclic peptide molecules, which has enriched the chemical space of complex peptides, and the related work was recently published in the Journal of the American Chemical Society.(DOI:
10.1021/jacs.2c04620
)。