In recent years, dioxazolone has attracted much attention as a precursor of nitrogenase. This compound can produce transition metal acyl-nitrogenase intermediates by removing CO2 under relatively mild reaction conditions. At present, the intermolecular C-H bond amidation and intramolecular C (SP3) - H bond amidation have been realized through this strategy.
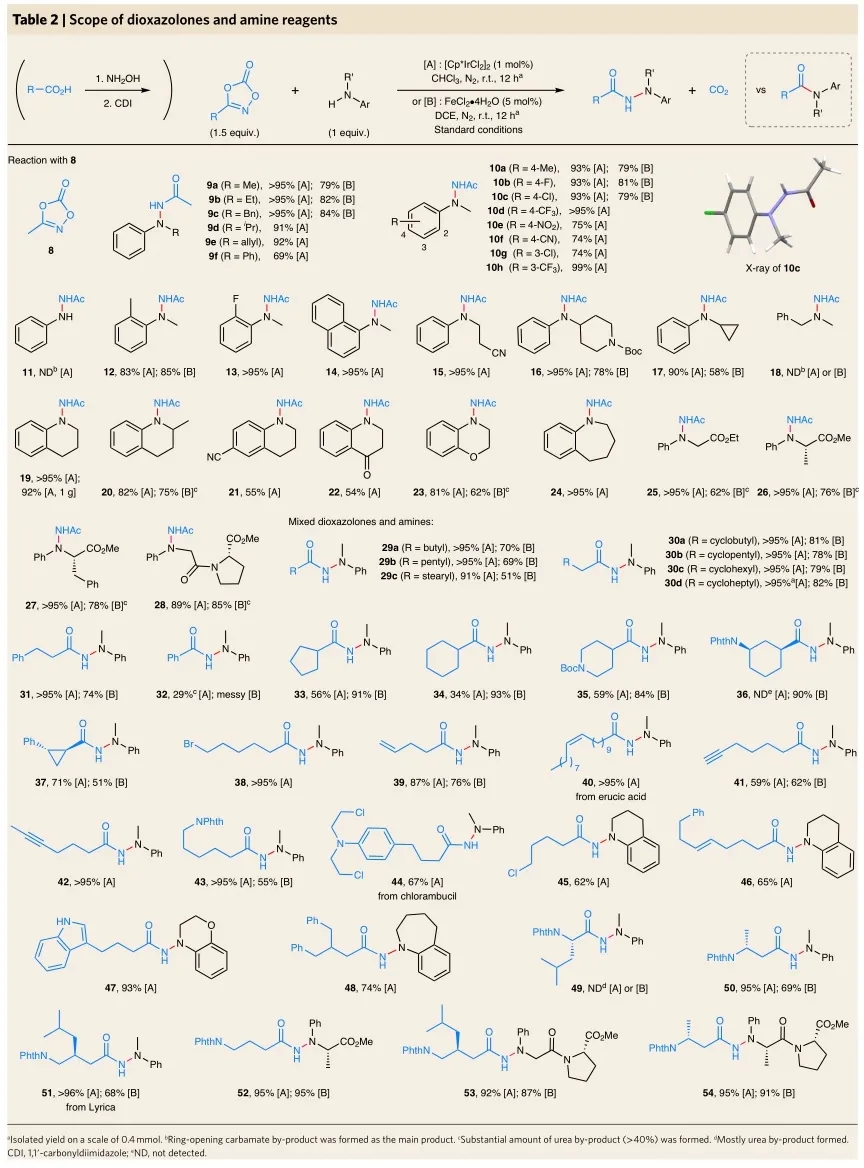
Recently, Professor Chen & He’s Group in Nankai University and Professor Sukbok Chang of KAIST have developed a series of N-arylalkyl hydrazides via N-N bond coupling between dioxazolone and aromatic amines based on the easily available precursors of carboxylic acids and aromatic amines (Figure d). The mechanism study showed that IR-acyl-Nitrenoid intermediate had strong electrophilicity and could react with arylamines through Cl…HN bonds, thus it overcome Curtius rearrangement and intramolecular C-H bond amidation. It can be predicted that this method has important application value in synthetic chemistry, pharmaceutical chemistry and agricultural chemicals.
Read more:
Nitrene-mediated intermolecular N–N coupling for efficient synthesis of hydrazides
Hao Wang, Hoimin Jung , Fangfang Song , Shiyang Zhu , Ziqian Bai, Danye Chen, Gang He , Sukbok Chang , Gong Chen
Nat. Chem., 2021, DOI: 10.1038/s41557-021-00650-0