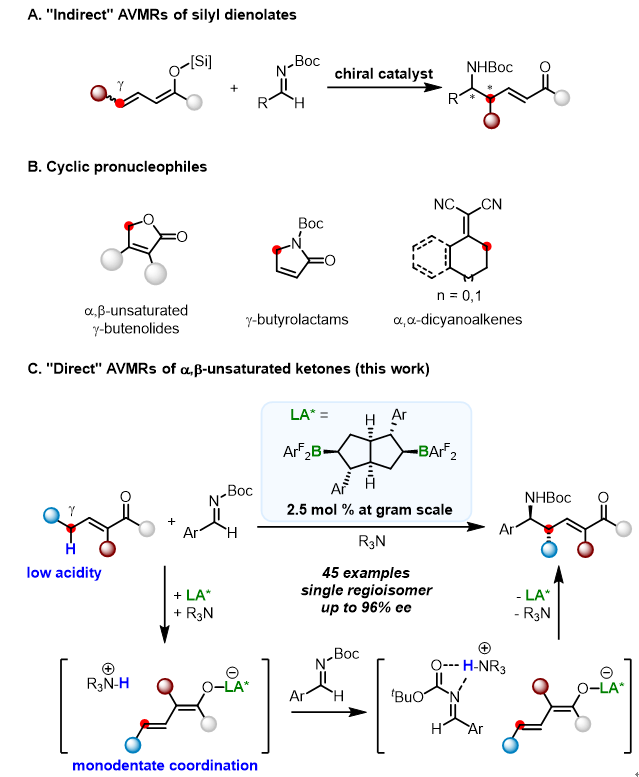
Professor Xiaochen Wang’s Group have achieved efficient synthesis of optically active unsaturated δ- Amino- α, β- unsaturated carbonyl derivatives by acyclic α, β- unsaturated ketone with high regioselectivity, diastereoselectivity and enantioselectivity under the catalyst of chiral diborone. In the reaction process, boron catalyst acts as a strong Lewis acid to coordinate with carbonyl group, thus enhancing the substrate γ acidity. Under the synergistic action of organic amines, chiral boron linked conjugated enol intermediates and ammonium cation are formed; Then, conjugated enol intermediate attacks imine to form target product. Among them, imines are activated by ammonium cations, and chiral boron catalysts control the regioselectivity and stereoselectivity.
The reaction does not need auxiliary groups, which is the first case of non-cyclic reaction of α, β- Direct AVRMs with unsaturated ketone as substrate. More importantly, this work demonstrated the unique advantages of chiral boron catalysts in which their strong Lewis-acid can effectively activate monodentate and weakly coordinated ketone substrates, and their large steric hindrance makes it possible for remote chiral control. This work provides an important reference for the further study of chiral boron asymmetric catalysis. This work was recently published in J. Am. Chem. Soc., 2021. (DOI: 10.1021/jacs.1c00006)
Read more:https://pubs.acs.org/doi/abs/10.1021/jacs.1c00006