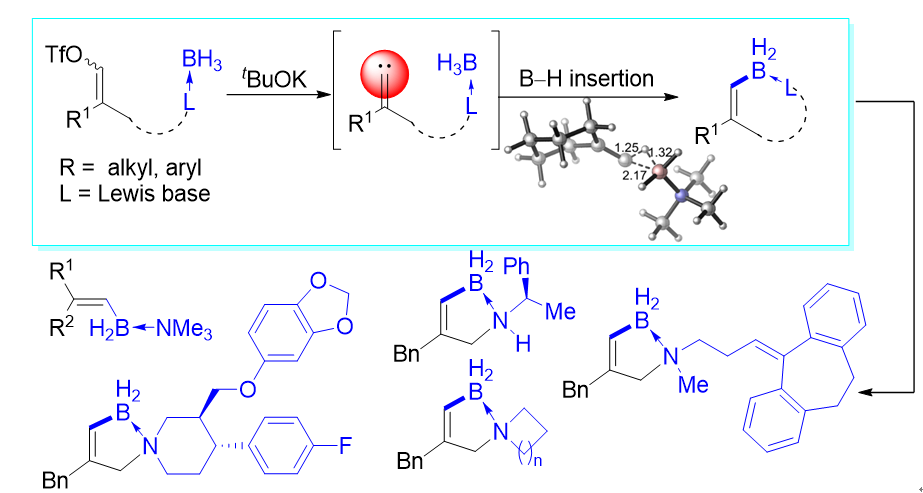
Recently, Prof. Zhou and Prof. Zhu group have developed a protocol for insertion of alkylidene carbenes into the B–H bonds of amine–borane adducts, enabling, for the first time, the construction of C(sp2)–B bonds by means of carbene-insertion reactions. Various acyclic and cyclic alkenyl borane–amine adducts were prepared from readily accessible starting materials in good to high yields and were subsequently subjected to a diverse array of functional group transformations. The unprecedented spiro B–N heterocycles prepared in this study have potential utility as building blocks for the synthesis of pharmaceuticals. Preliminary mechanistic studies suggest that insertion of the alkylidene carbenes into the B–H bonds of the amine–borane adducts proceeds via a concerted process involving a three-membered-ring transition state. This work now has been published on JACS (https://doi.org/10.1021/jacs.0c09596).