Carbon/silicon switching has been regarded as an innovative strategy for the development of new materials, drugs and pesticides. The structural units of tetralins and benzosuberanes at various oxidation levels are prevalent in numerous marketed drugs and natural products (Figure 1).
Figure 1
Here, Professor Dongbing Zhao’ group have developed a mechanistically unique ring‐expansion process of 4‐ and 5‐membered silacylces. With the aid of an alcohol, diverse 6‐, 7‐, and 8‐membered silacyles bearing a wide range of functional groups were synthesized. This method constitutes one of the most efficient and general routes for preparing sila‐tetralins and sila‐(benzo)suberanes, and could trigger the development of new drug‐like candidates due to the importance of the structural units of tetralins and benzosuberanes in medicinal chemistry and natural products(Figure 2). This work has been published on Angew. Chem. Int. Ed. (10.1002/anie.202001539).
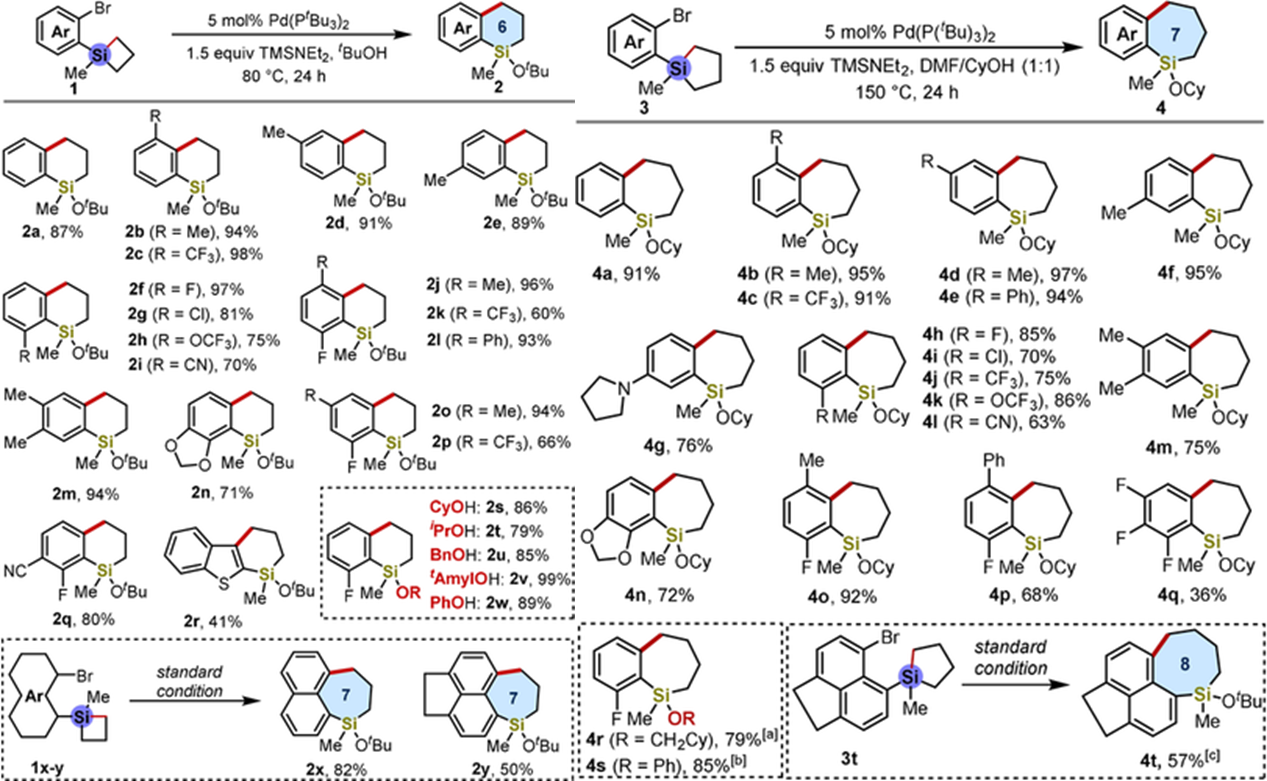
Figure 2