Cyclopropanes are versatile structural motifs in organic synthesis. Their high ring strain (ca. 115 kJmol-1 ) is often harnessed to cleave a C–C bond for further functionalizations on the disconnected carbon atoms. Common strategies to selectively open the cyclopropane ring include 1) the Lewis acid catalyzed activation of donor–acceptor (D-A) cyclopropanes and 2) transition-metal-mediated processes that often proceed by oxidative addition. The former approach requires the presence of electron-donating and -accepting groups to create sufficient electronic bias across the C–C bond. The latter approach usually utilizes a chelating group to capture transition metals to facilitate the process and/or direct transition metals towards the target C–C bond. However, C–C bond cleavage in simple alkyl- and aryl-substituted cyclopropanes has rarely been studied because their bonds are much less polarized, and they do not have a preinstalled functional group to interact with either Lewis acids or transition metals.
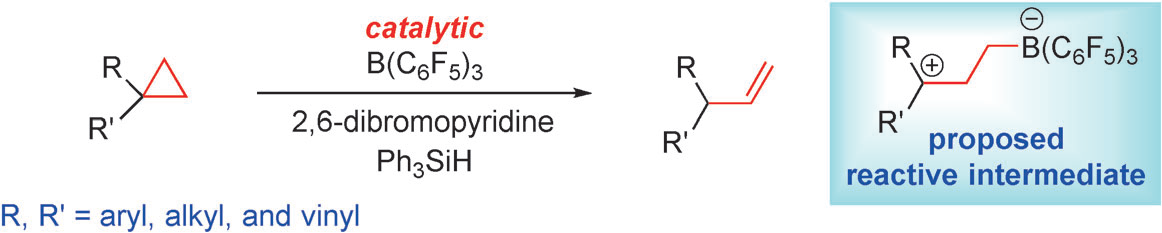
Prof. Xiaochen Wang have developed a catalytic cyclopropane ring-opening reaction that provides olefins by B(C6F5)3 - mediated C-C bond cleavage. Catalytic amounts of B(C6F5)3 promote the ring opening and subsequent isomerization of a series of unactivated cyclopropanes to afford terminal olefins in good yields when a hydrosilane and 2,6-dibromopyridine are employed as additives.This reaction is compatible with a broad range of unactivated cyclopropanes that are difficult to activate by other Lewis acids or transition metals.
Link: http://onlinelibrary.wiley.com/doi/10.1002/anie.201700864/full