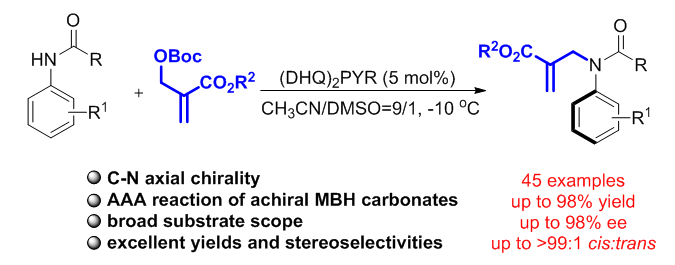
A highly efficient method to access axially chiral anilides through asymmetric allylic alkylation reaction with achiral Morita–Baylis–Hillman carbonates by using a biscinchona alkaloid catalyst was reported.Through the atroposelective approach, a broad range of axially chiral anilide products with different acyl groups, such as substituted phenyl, naphthyl, alkyl, enyl, styryl and benzyl, were generated with very good yields, moderate to excellentcis:transratios and good to excellent enantioselectivities.The reaction can be scaled up, and thesynthetic utility of axially chiral anilides was proved by transformations. Moreover, the linear free energy relationship analysiswas introduced to investigate the reaction.
Read More:https://pubs.acs.org/doi/pdf/10.1021/jacs.8b06014.