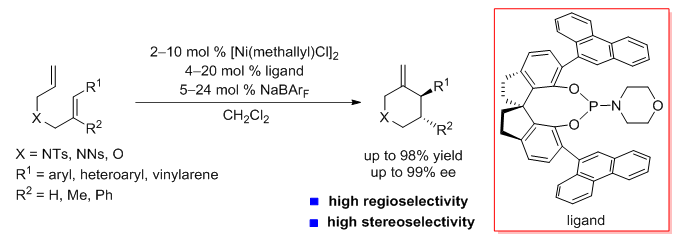
Chiral N- and O-heterocyclic moieties are ubiquitous in natural products, pharmaceuticals, and agrochemicals, and the development of efficient methods for their construction has attracted considerable attention in organic synthesis. Transition-metal-catalyzed enantioselective cyclization of N- and O-tethered unsaturated substrates is a straightforward, 100% atom-economical approach to chiral N- and O-heterocycles. Enantioselective cyclizations of N- and O-tethered enynes and allenynes have been achieved with chiral transition metal catalysts. In contrast, enantioselective cyclizations of N- and O-tethered dienes are rare. Herein, we report the first highly enantioselective intramolecular hydroalkenylation of N- and O-tethered 1,6-dienes mediated by a nickel catalyst modified with monodentate chiral spiro phosphorus ligands. This reaction provides six-membered-ring N- and O-heterocycles with excellent regioselectivity and enantioselectivity. Some of the reaction products are core structures of chiral drugs and natural products. The catalyst developed in this study represents one of the few catalysts for highly enantioselective cyclization of unconjugated dienes and has high potential for application in other cyclization reactions. (J. Am. Chem. Soc. 2018, 140, DOI: 10.1021/jacs.8b04703)