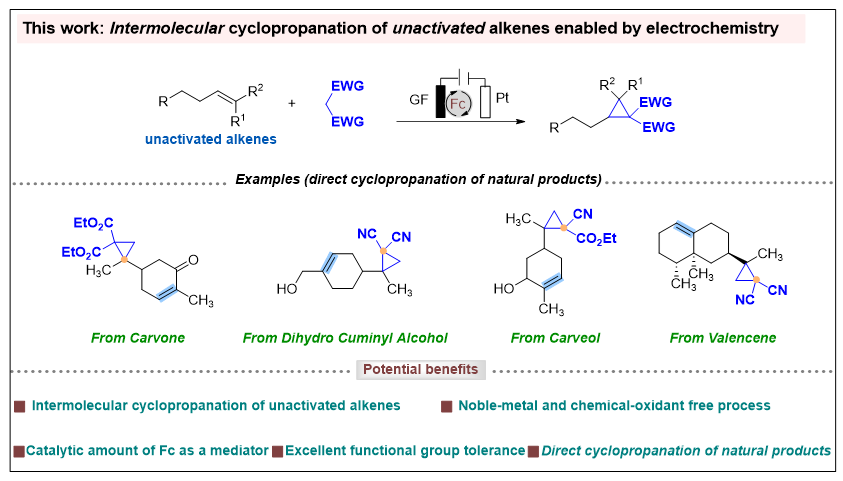
Cyclopropane, the smallest cycloalkane, is a basic structural element in a wide range of natural products and bioactive molecules. Consequently, the synthesis of cyclopropanes has consistently been of high interest in organic synthesis. However, their preparation is challenging due to their high strain energy, which renders them susceptible to unexpected ring opening. Despite this challenge, tremendous efforts from numerous chemists have paved the way forward to the synthesis of cyclopropanes. The typical methods include Simmons–Smith, Kulinkovich, and Corey–Chaykovsky reactions, transition metal-catalyzed alkenes cyclopropanation reactions (usually with diazoalkanes). Although the significant contributions of these strategies to the formation of cyclopropanes, there remain limitations, such as the stability of the products, the use of highly active/dangerous reagents, limited substrates, and/or product diversity. Concurrently, active methylene compounds are widely used in synthetic chemistry due to their ease of handling and ready availability. The combination of active methylene compounds and alkenes could offer a direct and attractive route to synthesize cyclopropanes. However, the direct and general method for cyclopropane derivatives synthesis from unactivated alkenes and methylene compounds has thus not been well elucidated. Thus, the development of straightforward cyclopropanation reactions from readily available starting materials under mild conditions, that are compatible with versatile functional groups, remains challenging and in high demand.
Recently, Youai Qiu’s group have successfully developed an electrochemical cyclopropanation reaction of unactivated alkenes and methylene compounds with Fc as a cheap and accessible mediator. The method demonstrates a broad substrate scope and good functional group tolerance, thus providing a convenient and efficient route for the installation of a cyclopropane unit in the late-stage functionalization of natural products and direct cyclopropanation of natural products, which would be helpful in drug discovery. The mechanistic studies offer persuasive and valid evidence to support the proposed mechanism involving Cp2Fe-mediated generation of C-centered radicals from methylene compounds. It is anticipated that this green and environmentally friendly method will find further applications in organic synthesis, pharmaceutical, and material science. Relevant achievements were published in Angew. Chem. Int. Ed., 2025. DOI: 10.1002/anie.202425634.