σ-Bond cross-exchange reactions that involve cleavage of two different single bonds and their subsequent exchange have gained much attention because it would significantly streamline a pathway to construct organic skeletons. Nonetheless, it is considerably more difficult in contrast to the well-known widespread alkene/alkyne metathesis due to the relatively lower reactivity of nonpolar σ-bonds and the difficulty in controlling the reaction direction and product distribution.
Murakami and Dongbing Zhao’s group demonstrated that σ-bond cross-exchange reaction can effectively act as a ring expansion strategy associating with the thermodynamic driving force from ring strain-release, thereby providing conceptually new approach to specific cyclic compounds, which are otherwise inaccessible. However, examples are still very rare. This probably stems from the challenges to distinguish the reactivity of two strained rings, mediate the cleavage of two distinct chemical bonds, suppress the undesired but well-documented preferential self-dimerization and/or decomposition of strained rings and ensure the high site-selectivity. Until now, only C-C/C-Si bond exchange4 as well as C-C/C-N bond exchange in two different strained rings have been successfully realized with high selectivity. Thus, the highly selective metathesis of other single bonds between two strained rings remain highly sought after and could likewise have a broadly beneficial impact on ring construction in the molecular sciences.
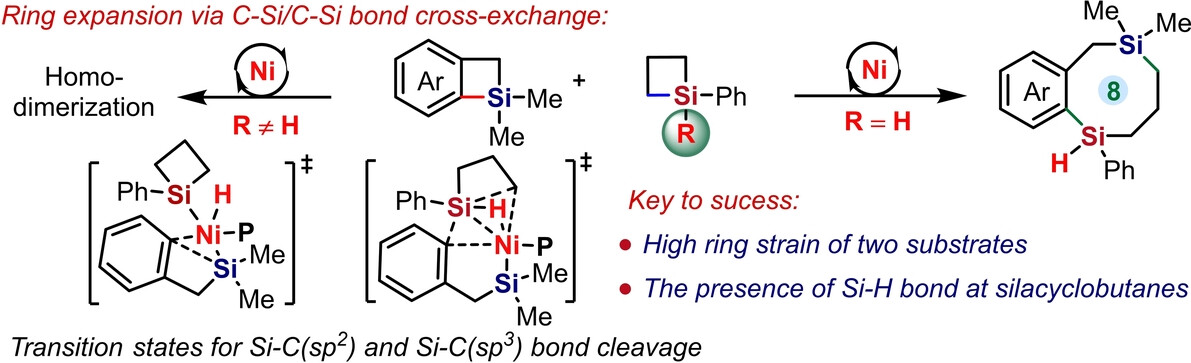
Recently, Dongbing Zhao’s group have developed the first intermolecular σ-bond exchange reaction of two different C-Si σ-bonds with high cross-redistribution selectivity by using the Ni-catalytic system. The four-membered benzosilacyclobutenes and silacyclobutanes are merged into diverse benzoannulated 8-memebered disila-carbocycles in an atom-economical manner. This protocol offers a concise access to cyclic compounds bearing two silicon atoms at the ring junction, which are otherwise inaccessible. Mechanistic studies confirmed that the C(sp2)-Si bond cleavage of benzosilacyclobutenes proceeds through oxidative addition and the C(sp3)-Si bond cleavage of silacyclobutane underwent a highly concerted process under the reaction. The presence of hydrogen on silicon of silacyclobutanes and the high ring strain for both of two substrates are critical to ensure the desired reactivity and selectivity. Relevant achievements were published in Angew. Chem. Int. Ed., 2024, DOI: 10.1002/anie.202319187.