Carbenes stand as pivotal intermediates in organic synthesis, facilitating a diverse array of transformations alongside carbocations, carbanions, and carbon radicals. Over the years, efforts to develop new classes of metal carbenes, particularly through catalytic formation strategies, have propelled the advancement of carbene chemistry. This progression not only amplifies the structural diversity of carbene-transfer products but also paves the way for the discovery of novel carbene-transfer reactions. In recent decades, researchers have leveraged various carbene precursors to establish asymmetric transfer reactions involving carbenes substituted with main-group elements such as C, P, S, and Si. These efforts, catalyzed by metal complexes or organocatalysts, have spawned versatile platforms for synthesizing a wide array of chiral molecules.
Boryl groups, especially tricoordinate boronic or boronate groups, manifest significant utility. Boronic acids and their derivatives excel in facilitating the stereoselective construction of various chemical bonds, finding applications in the self-assembly of nanomaterials, hydrogels, and saccharide sensors due to their reversible reactions with nucleophiles and hydrogen bond formation capabilities. Notably, boronic acids have also found a place in medicinal chemistry, acting as bioisosteres of carboxylic acids in a range of applications, including the formulation of FDA-approved tumor therapies such as ixazomib and bortezomib. This background sets the stage for the promising potential of α-boryl carbenes, a hybrid of carbene and boryl groups, as promising intermediates in the construction of organoborons.
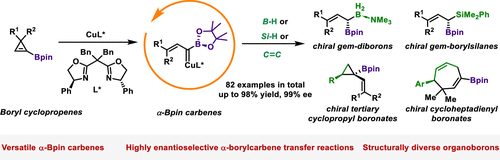
Recently, Shou-Fei Zhu’s group have devised a strategy for utilizing boryl cyclopropenes as precursors to synthesize a range of Bpin-substituted carbenes, thereby enabling a series of highly enantioselective α-boryl carbene-transfer reactions. These reactions, orchestrated by a copper/bisoxazoline complex, grant streamlined access to an array of structurally unique chiral organoborons, including chiral allylic gem-diborons, chiral allylic gem-borylsilanes, chiral tertiary cyclopropyl boronates, and chiral cycloheptadienyl boronates. These compounds have proven to be of significant synthetic utility. Mechanistic investigations suggest that the rate-determining step in this process is the opening of the cyclopropene ring. A plausible chiral model, elucidating the selective Re-face attack observed across all reactions, has been established, potentially serving as a blueprint for other enantioselective reactions involving bisoxazoline ligands. This approach paves the way for the exploration and development of additional functional chiral organoborons, leveraging the rich potential of carbene chemistry. Relevant achievements were published in J. Am. Chem. Soc., 2024, DOI: 10.1021/jacs.3c14766