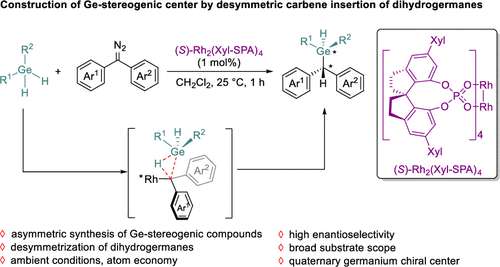
Chiral molecules play a significant role in the areas of pharmaceuticals, agrochemicals, and functional materials. Most chiral molecules contain a tetrahedral carbon atom that is connected to four different groups. Recently, chiral compounds having an asymmetric tetra-substituted main group element, such as sulfur, phosphorus, silicon, and boron have attracted increasing interest due to their promising biological and material properties. Germanium, another important main group element in Group 14 of the periodic table, has empty 3d orbitals and is prone to forming hypervalent intermediates (5- and 6-coordinated complexes). And germananes containing Ge–H bonds also tend to form germanium free radicals. A variety of organogermanium compounds have been synthesized and applied in organic synthesis, catalysis, drug discovery, and material science. However, the enantioselective synthesis of chiral Ge-stereogenic germanes remains a challenge, with only one example reported by Nozaki, via a rhodium-catalyzed enantioselective [2 + 2 + 2] cycloaddition. Therefore, a general synthetic method to prepare chiral germanium-stereogenic compounds is highly desired for exploring their functions.
Recently, Qi-Lin Zhou’s group have developed an enantioselective synthesis of Ge-stereogenic compounds has been achieved by the rhodium-catalyzed desymmetric Ge–H bond insertion of dihydrogermanes. The mild conditions endowed the reaction with excellent functional group tolerance. By this method, a variety of chiral germanes with Ge-stereogenic centers were synthesized with high yields and excellent enantioselectivities. Kinetic studies showed that the decomposition of the diazo compound is likely the rate-determining step. The remaining Ge–H bond of the chiral Germany products provides an opportunity for further derivatization. Relevant achievements were published in J. Am. Chem. Soc., 2024, DOI: 10.1021/jacs.3c14386.