Olefins are basic raw materials for the chemical industry and are widely used in the preparation of functional materials, pharmaceuticals, agrochemicals, and food additives. The configuration of the double bond of an olefin usually affects the selectivity of its reactions. Classical olefination methods typically produce mixtures of E/Z isomers, which are generally difficult to separate. Therefore, the development of methods for stereoconvergent transformations of E/Z mixtures of olefins, that is, the preparation of products with a single stereoconfiguration, can be expected to reduce the difficulty and cost of synthesis. However, only a few examples of stereoconvergent transformations have been reported, and the origin of stereoconvergence is not fully understood. Therefore, the development of new methods for the stereoconvergent transformation of olefins and elucidation of the mechanism of stereoconvergence would be of great scientific value and practical significance. Allylsilanes have proved to be one of the most versatile of the silicon-containing carbon nucleophiles, often showing high levels of stereocontrol during their attack on cationic electrophiles. Thus, it is important to develop efficient methods for the synthesis of allylsilane with high regioselectivity and stereoselectivity. However, most of these methods produced the thermodynamically more stable (E)-allylsilanes, while the synthesis of (Z)-allylsilanes still remains challenging.
Recently, Shou-Fei Zhu’s group reported a method for iron-catalyzed stereoconvergent 1,4-hydrosilylation reactions of E/Z mixtures of readily available conjugated dienes with various substitution patterns (Scheme 1). The method allowed the preparation of a series of synthetically important Z-allylsilanes with high regioselectivity and exclusive stereoselectivity. The method developed in this study uses readily available raw materials, has a wide substrate scope and good functional group compatibility, proceeds under mild conditions, and shows high regio- and stereoselectivity. An in-depth study of the mechanism indicates that a well-defined formal Fe(0) species initiates the two-electron redox catalytic cycle. Both isomers of the conjugated diene coordinate with the iron catalyst in a cis conformation and generate conformationally specific anti-π-allyl iron intermediates via ligand-ligand hydrogen transfer (LLHT), which ultimately determines the stereoselectivity of the reaction. The stereoconvergent mechanism disclosed in this study differs from the mechanisms of other related reactions mediated by radicals or metal-hydride species reported in other studies and can be expected to inspire the development of new stereoconvergent transformations of olefins as well as new iron-catalyzed reactions. Relevant achievements were published in Angew. Chem. Int. Ed., 2023, DOI: 10.1002/anie.202315473
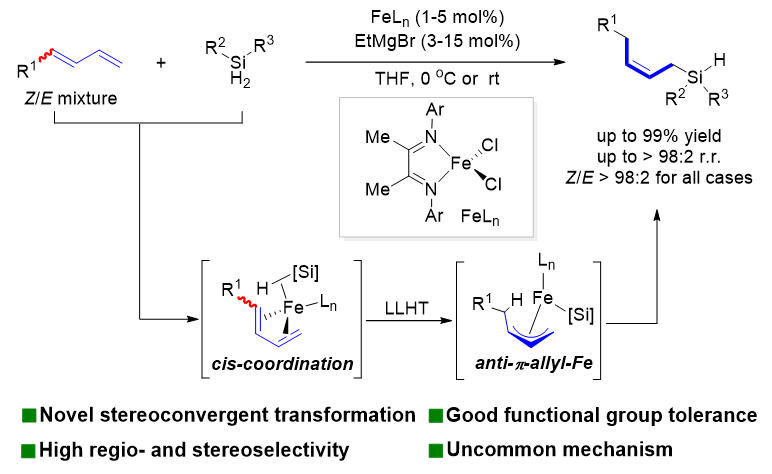
Scheme 1 Iron-catalyzed stereoconvergent 1,4-hydrosilylation of conjugated dienes.