Metal–ligand cooperativity has become a powerful tool for transition-metal-mediated small molecule activation and homogeneous catalysis. The investigation of the main-group element–ligand cooperativity is of great interest, offering promising opportunities to develop transition-metal free catalytic reactions. The geometric perturbation is a potent strategy to enhance the reactivity of the main-group compounds by tuning the energetic levels of frontier molecular orbitals and increasing the coordination space. In this regard, geometrically constrained main-group compounds with pincer-type ligands have excelled in cooperative bond activation. Besides, borylenes have attracted considerable interest as a result of their intriguing electronic structures and their unusual transition-metal-like reactivity.
Recently, Mo’s group reported the coordination of the bis(silylene)amido pincer ligand to boron(I), yielding a three-coordinate borylene (1) with a distorted T-shaped geometry. This compound (1) undergoes a single electron reduction with tris(pentafluorophenyl)borane (B(C6F5)3) to give the boron-centered radical pair [(SiNSi)B]•+[ B(C6F5)3]•–. Meanwhile, borylene 1 also leads to the facile cleavage of N–H, P–P, and C═O bonds in various substrates, which proceeds in a borylene–silylene cooperative fashion. In addition, 1 is an efficient catalyst for carbon dioxide reduction. These studies provide a broader opportunity for the design of novel main-group element–ligand cooperativity. Relevant achievements were published in J. Am. Chem. Soc. 2023, DOI: 10.1021/jacs.3c00949.
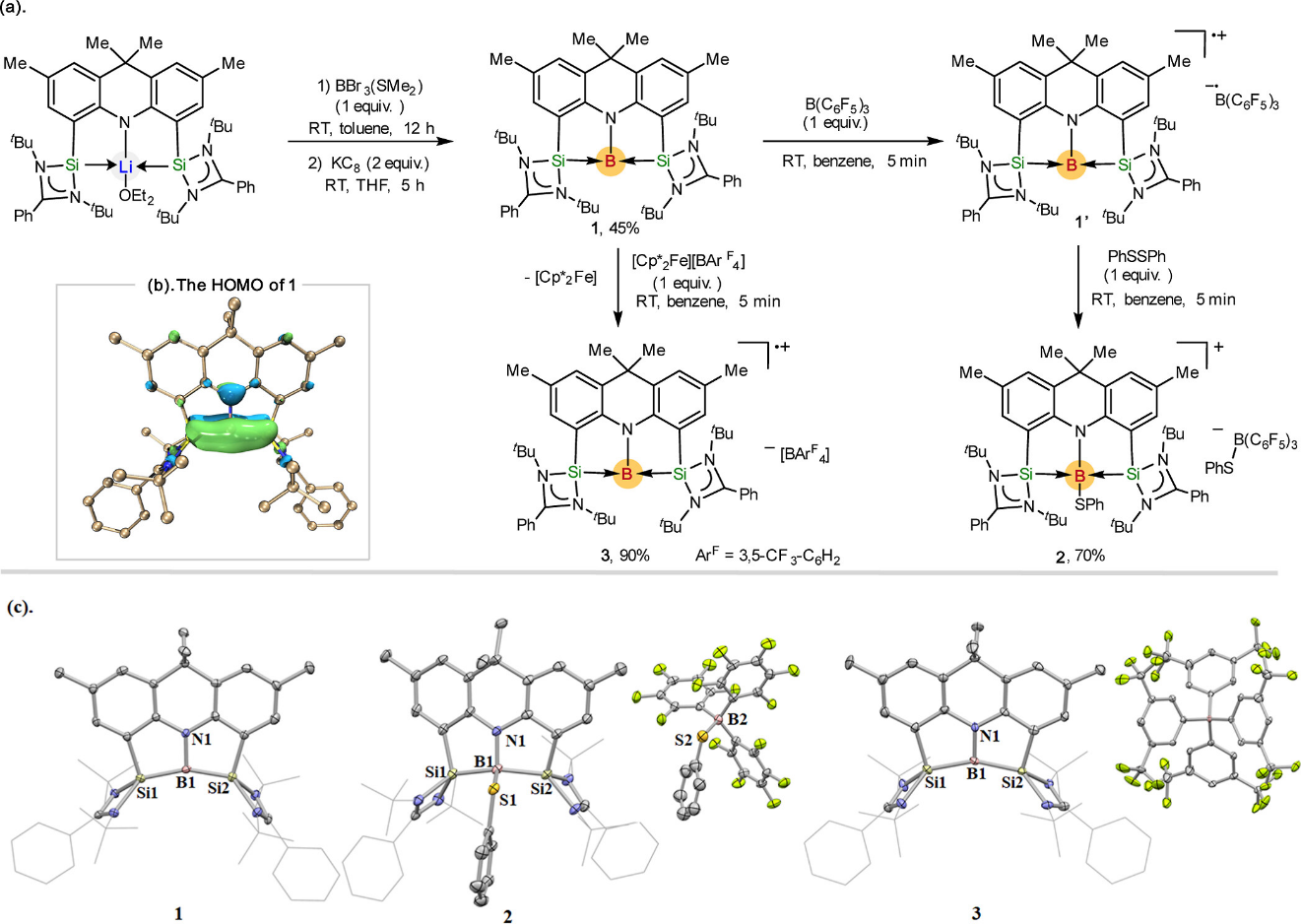
Scheme 1. (a) Synthesis of Bis(silylene)-Stabilized Borylene and Its Single Electron Transfer Reactivity; (b) HOMO of 1 Determined by DFT Calculations (Isovalue = 0.05); (c) X-Ray Crystal Structures of 1–3 at 50% Probability Ellipsoids