Aryl carboxylic acids are crucial scaffolds by themselves and key synthetic precursors in organic chemistry, material science, and pharmaceutical chemistry, making them highly valuable and attracting significant interest. Numerous novel methodologies to prepare aryl carboxylic acids have been reported, among which, direct carboxylation of aromatic compounds with CO2 has been regarded as an attractive approach. One representative strategy is to use pre-functionalized aromatic compounds, such as aryl boronates, aryl halides and organometallic substrates, including organolithium, Grignard reagents, and organozinc reagents. Meanwhile, electrosynthesis has been identified as an increasingly viable and ideal tool that avoids the use of stoichiometric amounts of redox reagents in organic synthesis. Thus, the combination of direct carboxylation with electrosynthesis has attracted considerable attention.
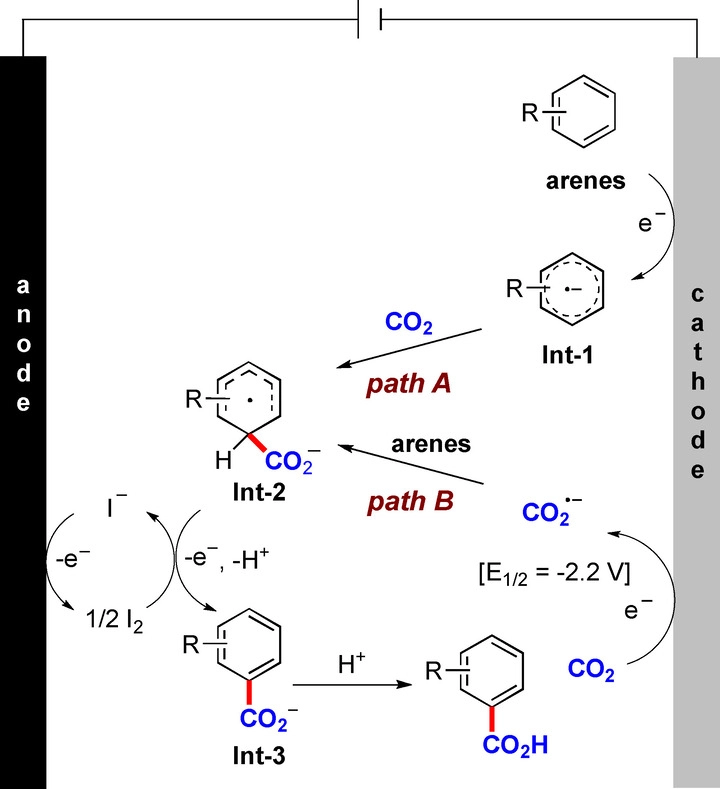
Recently, Youai Qiu’ group achieved a direct, metal-free, and site-selective electrochemical C−H carboxylation of arenes by reductive activation using CO2 as the economic and abundant carboxylic source. The electrocarboxylation was carried out in an operationally simple manner with high chemo- and regioselectivity, setting the stage for the challenging site-selective C−H carboxylation of unactivated (hetero)arenes. The method benefits from being externally catalyst-free, metal-free and base-free, which makes it extremely attractive for potential applications. Relevant achievements were published in Angew. Chem. Int. Ed., 2022, DOI: 10.1002/anie.202214710.