Nitrile groups are present in various natural products, drugs, agrochemicals, and polymers. For drugs in particular, the nitrile group is an ideal ligand for binding to target proteins because of its high polarity, small size, linearity, and metabolic stability and its ability to accept hydrogen bonds. Moreover, nitriles can be readily transformed to carboxylic acids, aldehydes, ketones, amides, and amines. Therefore, methods for the installation of nitrile groups into organic molecules are highly useful.
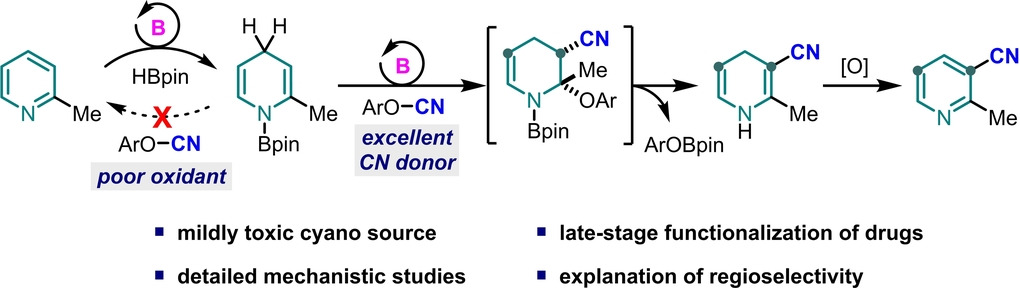
Recently, Xiaochen Wang’ group achieved the interposition cyanylation of pyridine by introducing cyanoelectrophilic reagents. Among them, the substitution reaction of dihydropyridine with cyanoelectrophilic reagents underwent an addition/elimination process, which was found for the first time in the series of studies. They studied the reaction mechanism in detail by means of control experiments and theoretical calculations and found that a combination of electronic and steric factors determined the regioselectivity of reactions involving C2-substituted pyridines. The method is suitable for late-stage functionalization of pyridine drugs. The low reduction potential of the electrophile and effective transfer of the nitrile group were found to be essential for the success of this method. Relevant achievements were published in Angew. Chem. Int. Ed., 2023, DOI: 10.1002/anie.202216894.