The introduction of fluoride functional groups into drug molecules can improve the cell membrane permeability, target specificity and metabolic stability of drugs. Trifluoromethylthio (SCF3) is one of the most lipophilic functional groups. The hydrogen atom of difluoromethylthio (SCF2H) has weak acidity and can form hydrogen bonds with enzymes. At present, several drugs containing SCF3 and SCF2H have been approved for marketing.
Xiaochen Wang 's group has been committed to the research of organic boron catalytic chemistry. They previously established a new method for the C-H bond alkylation of pyridine at C3 position through the dihydropyridine intermediate generated by borane catalyzed pyridine borohydride. Recently, based on this work, their group successfully realized the trifluoromethyl thiolation and difluoromethyl thiolation of pyridine at C3 position. This method has a wide range of substrates, high functional group compatibility, and is suitable for post modification of drug molecules. It has high application value. Moreover, this work indicates the potential applicability of other heteroatom electrophiles in this strategy.
Relevant achievements were recently published in J. Am. Chem. Soc., DOI: 10.1021/jacs.2c06776
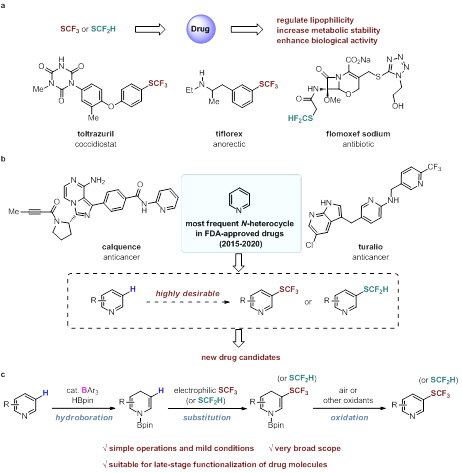
C3-trifluoromethylthiolation and difluoromethylthiolation of pyridine.