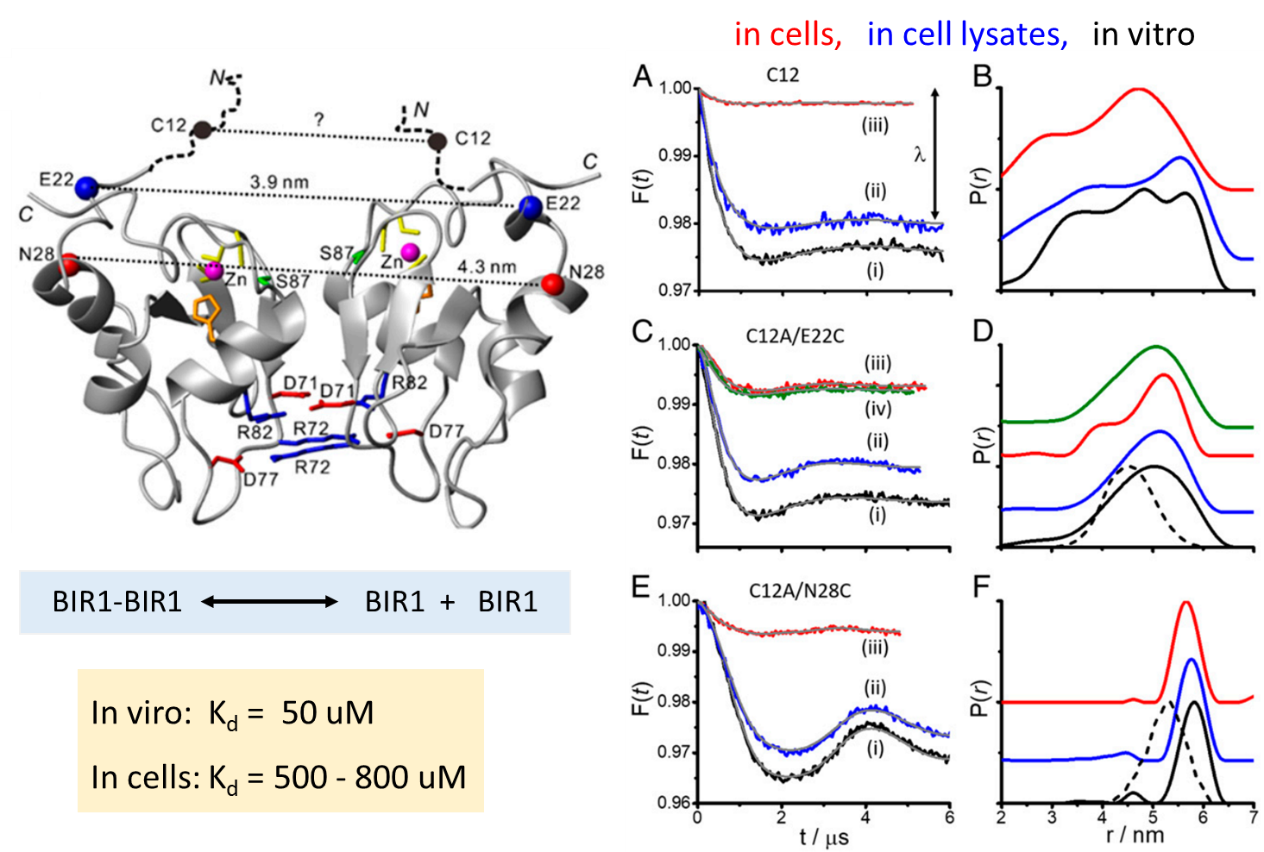
The question how does the intracellular environment affect the structure, dynamics, and stability of a protein and protein–protein interactions has been a center of interest in the context of understanding proteins function in their native environment. Recently, Prof. Xuncheng Su’s group addressed this question by exploring the conformational space and the stability of a homodimeric protein complex in human cells as compared to in dilute buffer solution and cell lysates. We showed that the dimer is destabilized in the cells, which is opposite to the theoretical predictions. Based on complementary in vitro measurements, we suggest that the destabilization originates from a combination of the crowding and shape of the dimer, the higher ionic strength in the cell, and potential attractive chemical interactions. This work has been published on Proc. Natl. Acad. Sci. USA, 2020, 117, 20566-20575.