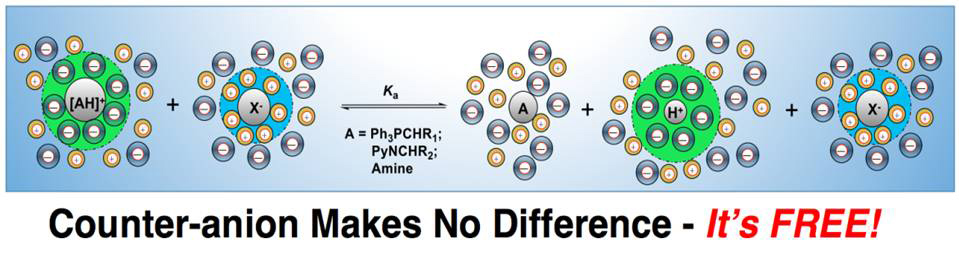
Absolute pKas of selected salts with different counter-anions were measured with high precision in four aprotic ionic liquids (AILs), which enables a detailed examination of solvation effect of ILs on salts. Interestingly, the counter-anions of the ylide precursor salts, protic amine, and phenol salts of this study, though differing dramatically in size and electron dispersion, were found to have no effect on the respective pKas of the substrates. This indicates that the ionic species generated upon acidic dissociation of the salts in weakly polar AILs of low dielectric constant (ε: 10–15) are not ion-paired, or in other words, behave like “free ions” as if in strongly dissociating molecular solvents of high polarity (e.g., DMSO). This suggests that the widely assumed ion-pairing phenomenon, an issue of much debate, is not important in the AILs under our experimental conditions, presenting a typical “ionic-liquid effect” on the solvation of charged species in AILs.
Link: http://pubs.acs.org/doi/abs/10.1021/jacs.6b02607.