Organofluorine compounds are extremely rare in nature, as only five natural fluorinases have been discovered.Fluorine substitution is becoming increasingly popular because it can improve the properties of different classes of functional molecules, including pharmaceuticals, agrochemicals, and materials.For example, the incorporation of fluorine atoms into pharmaceuticals can increase metabolic stability, lipophilicity, and overall biological activity, and approximately 20 % of commercially available pharmaceuticals contain at least one fluorine atom. Therefore, the development of new methods for the introduction of fluorine into small molecules has received extensive attention.Organosilanes are useful intermediates in organic synthesis and reactions, such as the Hiyama coupling reaction. However, the fluorination of organosilanes with fluoride ions is uncommon because fluoride ions interact with silicon atoms to form Si−F bonds, which have high bond dissociation energies.
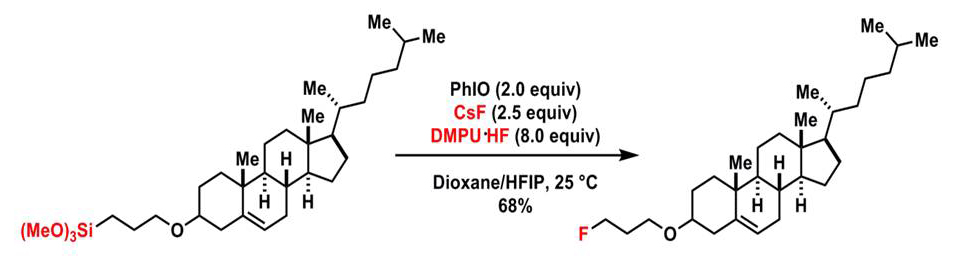
Prof. Pingping Tang's group have developed thefirst example of a hypervalent iodine(III)-mediated oxidative fluorination of alkylsilanes by fluoride ions without the use of transition metals is demonstrated. This reaction is operationally simple, scalable, and proceeds under mild reaction conditions. Mechanistic studies suggest the involvement of a single-electron transfer resulting from the interaction of an organopentafluorosilicate and aryliodonium difluoride, which were generated in situ from the corresponding alkylsilane and iodosobenzene, respectively, in the presence of fluoride ions.
Link: http://onlinelibrary.wiley.com/doi/10.1002/anie.201609741/full