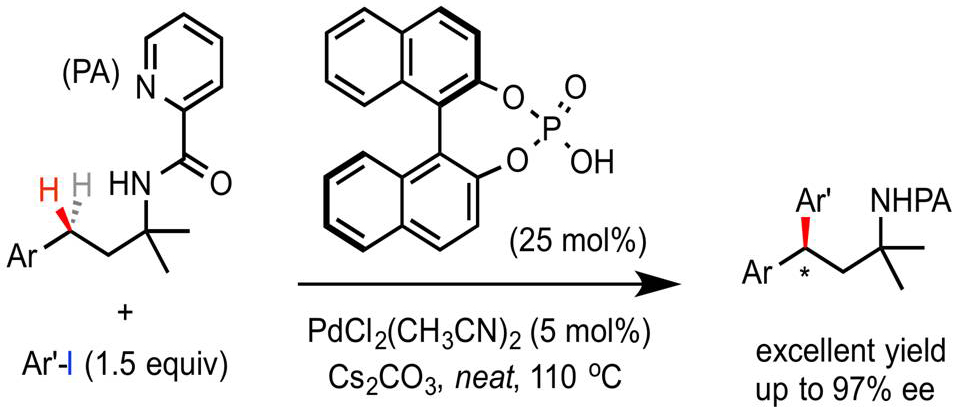
Prof. Gong Chen ang Prof. Gang He report an enantioselective palladium(II)-catalyzed bidentate auxiliary directed benzylic C−H arylation reaction of PA-derivatized alkyl amines with aryl iodides. Complementary to the previously reported BINOL phosphoric amide mediated enantioselective C−H arylation of aminoquinoline-derivatized alkyl carboxylic acid substrates, this reaction provides the first example of enantioselective γ-C−H arylation of PA-derivatized alkyl amines with up to 97 %ee. The combination of a BINOL phosphate ligand and Cs2CO3 base under solvent-free conditions is essential to achieve high enantioselectivity. Mechanistic studies suggest that a cesium–phosphate complex might be involved in the stereodetermining C−H palladation step. Additional mechanistic investigations and the development of new chiral phosphate ligands are currently under investigation to broaden the scope of the enantioselective palladium-catalyzed bidentate auxiliary directed C−H functionalization.
Link: http://onlinelibrary.wiley.com/doi/10.1002/anie.201609337/full.