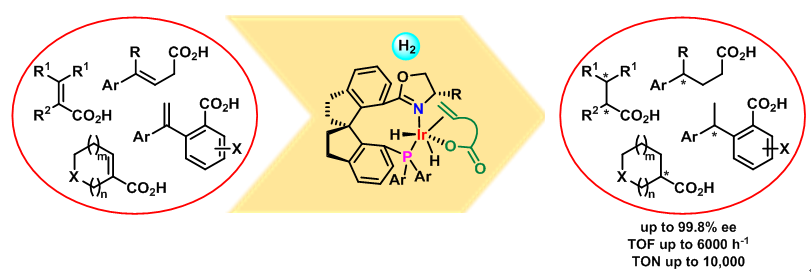
Chiral carboxylic acid moieties are widely found in pharmaceuticals, agrochemicals, flavors, fragrances, and health supplements. Although they can be synthesized straightforwardly by transition-metal-catalyzed enantioselective hydrogenation of unsaturated carboxylic acids, but because the existing chiral catalysts have various disadvantages, the development of new chiral catalysts with high activity and enantioselectivity is an important, long-standing challenge. Ruthenium complexes with chiral diphosphine ligands and rhodium complexes with chiral monodentate or bidentate phosphorus ligands have been the predominant catalysts for asymmetric hydrogenation of unsaturated acids. However, the efficiency of these catalysts is highly substrate-dependent, and most of the reported catalysts require a high loading, high hydrogen pressure, or long reaction time for satisfactory results.
Recent studies from prof. Zhou's group have revealed that chiral iridium complexes with chiral spiro phosphine-oxazoline ligands and chiral spiro phosphine-benzylamine ligands exhibit excellent activity and enantioselectivity in the hydrogenation of α,β-unsaturated carboxylic acids, including α,β-disubstituted acrylic acids, trisubstituted acrylic acids, α-substituted acrylic acids, and heterocyclic α,β-unsaturated acids. On the basis of an understanding of the role of the carboxy group in iridium-catalyzed asymmetric hydrogenation reactions, they developed a carboxy-group-directed strategy for asymmetric hydrogenation of olefins. Using this strategy, they hydrogenated several challenging olefin substrates, such as β,γ-unsaturated carboxylic acids, 1,1-diarylethenes, 1,1-dialkylethenes, and 1-alkyl styrenes in high yield and with excellent enantioselectivity. All these iridium-catalyzed asymmetric hydrogenation reactions feature high turnover numbers (up to 10,000) and turnover frequencies (up to 6000 h-1), excellent enantioselectivities (greater than 95% ee with few exceptions), low hydrogen pressure (<12 atm), and operational simplicity. these features make chiral iridium catalysts superior or comparable to well-established chiral ruthenium and rhodium catalysts for asymmetric hydrogenation of unsaturated carboxylic acids. A number of chiral natural products and pharmaceuticals have been prepared by concise routes involving an iridium-catalyzed asymmetric hydrogenation of an unsaturated carboxylic acid as a key step.
As part of a mechanistic study of iridium-catalyzed asymmetric hydrogenation of unsaturated acids, They isolated, for the first time, the migratory insertion intermediate in the iridium-catalyzed asymmetric hydrogenation of olefins, and this result strongly supports the involvement of an Ir(III)/Ir(V) catalytic cycle. The rigid, bulky scaffold of the chiral spiro P,N-ligands of the catalysts not only prevents them from undergoing deactivating aggregation under the hydrogenation conditions but also is responsible for the efficient chiral induction. The carboxy group of the substrate acts as an anchor to ensure coordination of the substrate to the iridium center of the catalyst during the reaction and makes the hydrogenation proceed smoothly.
Read more:
Shou-Fei Zhu*, Qi-Lin Zhou*, Iridium-catalyzed asymmetric hydrogenation of unsaturated carboxylic acids, Acc. Chem. Res. 2017, 50, 988‒1001. http://pubs.acs.org/doi/abs/10.1021/acs.accounts.7b00007