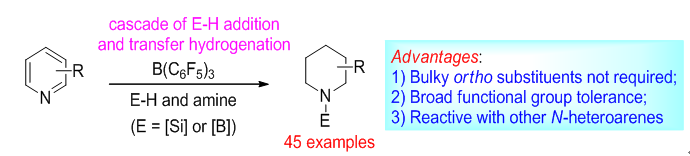
The catalytic reduction of pyridines is a research subject of significant interest to the synthetic community since the reduced products, including piperidines and partially reduced azacyclic compounds, are prevalent building blocks for the syntheses of many bioactive alkaloids and commercial drugs.Extensive efforts have been made for the development of transition metal catalyzed hydrogenations of pyridines using various heterogeneousand homogeneouscatalysts. However, harsh reaction conditions and the use of precious metals were often required for efficient catalytic turnover. These requirements have limited the substrate scope and the practical utility of these methods. In contrast, with the development of frustrated Lewis pair chemistry,several studies of metal-free organoborane-catalyzed pyridine reductions have appeared in the literature over the past few years, including hydrogenations developed by the groups of Stephan,Du,and Crudden,the 1,4-hydroboration developed by the group of Wang,the cascade hydrosilylation developed by the group of Chang,and the transfer hydrogenation developed by the group of Du.Despite these advances, there are still drawbacks: 1) For hydrogenation and transfer-hydrogenation reactions,the presence of bulky ortho substituents on the pyridine ring was necessary to circumvent the deactivation of catalysts by the coordination of the nitrogen atom; 2) Unsaturated functional groups such as alkenes, esters, ketones, nitriles, and nitro groups are rarely compatible because they were prone to reduction.
Xiaochen Wang's group have developed a B(C6F5)3-catalyzed metal-free pyridine reduction strategy by a cascade process of dearomative hydrosilylation (or hydroboration) and transfer hydrogenation. The broad functional-group tolerance provides easy access to an array of diversely functionalized piperidines and tetrahydropyridines which are valuable building blocks in synthesis. Its suitability for use in the reduction of other N-heteroarenes has also been demonstrated.
Link: http://onlinelibrary.wiley.com/doi/10.1002/anie.201702304/full