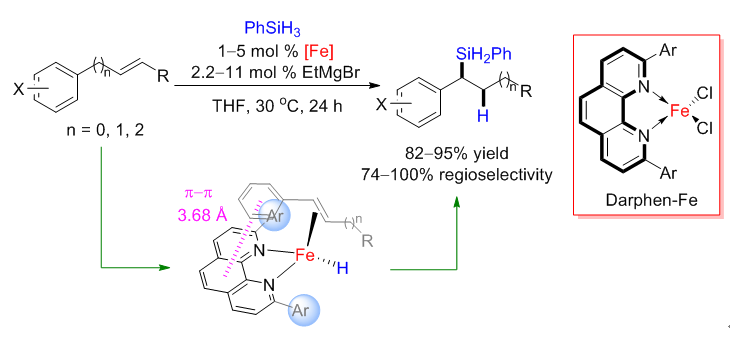
Transition-metal-catalyzed alkene hydrosilylation is one of the most important homogeneous catalytic reactions, and the development of methods that use base metals, especially iron, as catalysts for this transformation is a growing area of research. However, the limited number of ligand scaffolds applicable for base-metal-catalyzed alkene hydrosilylation has seriously hindered advances in this area. Herein, we report the first use of 1,10-phenanthroline ligands in base-metal catalysts for alkene hydrosilylation. In particular, iron catalysts with 2,9-diaryl-1,10-phenanthroline ligands exhibited novel reactivity and selectivity for hydrosilylation of alkenes, including unprecedented benzylic selectivity with internal alkenes, unique Markovnikov selectivity with terminal styrenes and 1,3-dienes, and excellent activity toward aliphatic terminal alkenes. We proposed catalytic cycles based on high spin Fe(I) catalysts, which can be support by a set of control experiments and computations. According to the mechanistic studies, the unusual benzylic selectivity of this hydrosilylation initiates from π-π interaction between the phenyl of the alkene and the phenanthroline of the ligand. This new ligand scaffold and its unique catalytic model open new possibilities for base-metal-catalyzed hydrosilylation reactions.
Read more:
Meng-Yang Hu, Qiao He, Song-Jie Fan, Zi-Chen Wang, Luo-Yan Liu, Yi-Jiang Mu, Qian Peng*, Shou-Fei Zhu*, Ligands with 1,10-phenanthroline scaffold for highly regioselective iron-catalyzed alkene hydrosilylation, Nature Commun. 2018, 9, 221.
https://www.nature.com/articles/s41467-017-02472-6